This is part two of Can Politicians Save US. Both are part of the Politics of Care forum. It would be great if you could print off these two posts and send to your local politician, asking what they figure on doing about some of these points.
Nine
Point Six in Can Politicians Save Us, mentions an Arms race and compared Drugs and Guns. But there are important differences between Drugs and Guns. With Guns, successive weapon systems trumped older ones because they were demonstrably more effective than what had gone before. This is not true of Drugs.
In the case of Drugs, it is the appearances of efficacy that trumps what has gone before. Unlike Guns, the latest Drug need not be better than the last Drug. Drugs are almost unique among modern technologies – Guns, Autos, Phones, Computers – in often being less effective and less safe than what has gone before.
A Drug is a Chemical plus Information that tells us how to use this chemical effectively and safely. The Chemicals were always dangerous and are not getting any safer.
Before the Fall of the Berlin Wall, the Information was largely accurate but now the greatest concentration of Fake Literature centres on the drugs our doctors give us.
In 1962, Pharmaceutical Companies were gifted a gadget that transforms poisons into sacraments and junk into life-saving remedies – the gadget is called a Randomized Controlled Trial (RCT).
RCTs are spun as offering Gold Standard Knowledge about Drugs but they are not knowledge. They are an algorithm put in place to help evaluate one, and one only, of the several thousand effects every drug has. To evaluate an effect in which some company has a commercial interest.
This money-tree effect may not be the commonest effect of a drug. In the case of SSRI antidepressants, numb genitals is the commonest effect. A mood benefit is pretty rare in comparison. But because RCTs hypnotize investigators to look at mood effects only, in SSRI trials they completely missed the numb genitals.
See The Fault Lies in Our Stars, Fawlty Stars and Does My Bias look Big in This.
Now, as outlined in Can Politicians Save Us, point 6, we have falling Replacement Rates in most Western countries.
RCTs transform Poisons into Sacraments. Sacraments offer Efficacy without Safety issues – although even the Vatican now supports Gluten-free Eucharists.
RCTs Greenwash Chemicals.
Combining RCTs in the Guidelines of Evidence Based Medicine (EBM) locks everyone in healthcare into a cage the way GDP locks politicians into an economic cage. GDP reflects activity of a certain kind rather than activity we necessarily value. The activity includes cleaning up after the Exxon Valdez oil spill but doesn’t include pristine, plastic or oil free oceans.
In the same way the Drug Wrecks that come from administering Poisons with gay abandon, thinking they are Sacraments, has made treating the consequences of our treatments the leading form of health service activity.
If you are desperate for a metric – create one that flags up the proportion of health service activity that stems from treatment induced injury and death. This may now be as much as 50% of all activity, stemming especially from treatments supposed to be ‘preventive’ that companies market as reducing health costs.
Ten
Point Six in Can Politicians Save Us, and Nine above, mention an Arms race and compare Drugs and Guns. With Guns, successive weapon systems trumped older ones because they were demonstrably more effective than what had gone before. This is not true of Drugs.
In the case of drugs its the Propaganda Techniques not the Pharmaceutical Technologies that persuade people to buy. There is no access to the data on drugs that shows how efficacious they actually are or how safe. The claims about what they do and don’t do are written up by ghostwriters.
Biden has just been blaming Facebook and Social Media platforms for vaccine misinformation. While the New England Journal of Medicine and JAMA and other leading journals are stuffed full of ghostwritten articles about on-patent medicines, articles that in some cases have been proven outright fraudulent and perhaps are all to some extent fraudulent, with the journals saying its not their job to guarantee the integrity of the data, without Joe mentioning this, its difficult to know what to call him – naïve or hypocritical or what – part of the Deep State perhaps?
The greatest concentration of Fake News on earth centres on the drugs our doctors give us.
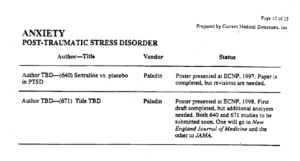
TBD in the slide above means To Be Determined. The document from which this slide comes was prepared in 1999. The articles on Zoloft studies for NEJM and JAMA had already been written by the ghostwriters – along with dozens of others. Zoloft was being sold for PTSD for which it was ineffective and unsafe, likely leading to the suicide of many US Veterans.
After the articles are written, pharmaceutical company marketing departments let the ghosts know which academic and clinical names are best suited to go on the authorship line. Best suited means who will be the best advocates for the drug.
Pfizer in this case had been advised by Paul Leber, ex-head of the CNS division of FDA, on how to get Zoloft which was ineffective, approved by FDA for PTSD, a condition Leber didn’t think was a real condition.
In response to a HealthCare rapidly deteriorating into health services, when things go wrong we are told that this is a problem of doctors not keeping to Guidelines. Its just the opposite. Doctors and increasingly nurses keep to the Guidelines more than ever before – because prescribers now get sacked if they stray from them.
Keeping to Guidelines cannot give us better Care if these are based on junk designed to sell the latest product with no regard as to whether the product is more effective or safer or better value for money. The Guidelines for PTSD endorsed the use of SSRIs like Zoloft.
Eleven
Require universities to tackle their academics who let their names be put on ghostwritten medical articles without having access to and without being able to share the full study data with other doctors and academics and ideally the wider public.
At present Guideline Makers, Regulators, Journals, Politicians, Medical Associations all say it is not their business to police the medical literature. Facebook operates to a much higher publishing standard than Medical Journals.
Anyone who wants to know more should read Paul John Scott’s all too real account of exactly what goes on. Whichever country you are in you can substitute in the names of many of the most prominent medical academics such as Siegfried Kasper, Professor of Psychiatry in Vienna. We are told we can trust these academics because they have over 1000 articles to their names.
Twelve
Behind this Fake Literature, there is an even deeper problem. There is no access to the data from clinical trials. No-one gets to see this. Remember drugs are not chemicals – they are chemicals that come with information about their use. Significant amounts of the information we get are fraudulent but in the absence of access to the data, this cannot be exposed.
- Regulators don’t see the data,
- Guideline Makers don’t see the data,
- The ‘Authors’ of papers reporting study results don’t see the data,
- The Ghosts who really write the papers don’t the data,
- The Doctor who might be treating you or your family hasn’t seen the data,
- If you are injured by a drug and want to find out more, you could not see the data even if you were Prime Minister, Chancellor or President.
The correspondence with regulators, Guideline Makers, Politicians, Medical Societies and others, supporting these claims is laid out in From Stephen O’Neill to the Crack of Doom.
When raising this problem with all these bodies I made it clear that it is not their job to police the medical literature. They now all parrot this response.
See Access to Clinical Trial Data, property rights, privacy rights and phoney rights
In honour of and Harvard/Boston where ghostwriting has been endemic and there is an understanding that no data will be asked for, a policy supported by successive Deans of Medicine like Jeff Flier, who presided over an orgy of shoddy links between a university and industry – see tweet in a series about ghostwriting and Harvard since deleted –
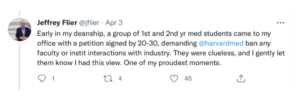
And the New England Journal of Medicine, who in 2009, made it clear they didn’t feel it was the journal’s responsibility to have check on the integrity of clinical trial data – see Philip Fine’s article HERE
Perhaps, its time for a Revolution or at least a Tea Party with the catchphrase:
No Prescription without Representation.
Thirteen
Change the consent forms for clinical trials.
Consent Forms began life in 1962 informing you that you were participating in research – you were often participating without knowing it before that.
Companies have since inserted phrasing telling us they will show our data to no-one. Reassured, we sign.
Companies now use these forms to block access to our data. There is no ethical, legal and in particular no scientific basis to refuse access – see Good Science or Good Business.
Our Signatures now put our families, friends and communities in a state of Legal Jeopardy – See Clinical Trials and Legal Jeopardy.
The data, which does not belong to companies, is being hidden for commercial reasons not out of any concern for the privacy of anyone who enters a trial.
There can be no argument about getting access to the data from premarketing healthy volunteer trials of drugs. These are run before drugs come on the market and in the case of the antidepressants revealed the suicidality, homicidality and permanent sexual dysfunction these drugs can cause. After marketing companies denied completely that these hazards exist and it took 15 years to get warnings about suicidality and we still don’t have the appropriate warnings about sexual function.
There are no issues of clinical confidentiality here. These are the trials that show what drugs are really causing – that allows pharmaceutical companies to game their later clinical trials in patients, where everything can be blamed on the illness being treated.
This is what an original consent form looked like.
Fourteen
It may take political support for Doctors to screw up the courage to ask companies for clinical trial data on a drug they are proposing to give you or your family. See Can Doctors Save Their Jobs and the World.
It may take political support for Doctors to screw up the courage to refuse to use guidelines – like the NICE Guidelines – that are not based on data – See The Nice Before Christmas.
If they are unwilling to do this, then doctors are like salt that have lost their saltiness – useless.
Like the Greek Women in Aristophanes drama Lysistrata, who stopped a war and the killing of Greeks by Greeks by refusing to make love to their men, so also pharmaceutical companies need doctors more than doctors need them.
Make it possible for doctors to refuse to tango with Pharma while they continue to kill and maim so many of your voters because they lack a backbone. Get them to do their job and your job will be done.

Remember No Prescription without Representation – or perhaps that should be We will not be Dicktated To.
Fifteen
Mobilise us, your voters, at risk of being killed or maimed when we visit our doctors, to ask if our doctor has access to the data on the drug they are proposing to prescribe to us. Empower us to declare a preference for being given medicines whose data is accessible -there will always be older, better and less expensive drugs that can be used.

See If you are going to Treat Patients, Man Up and Scaremongers of the World Unite.
Sixteen
Require doctors to report adverse events their patients suffer on treatment to pharmaceutical companies leaving the doctor’s name on these events and indicating a willingness to be cross-examined about the event. At present doctors only report 1 in 100 serious adverse events, primarily to regulators, and do not expect to be interrogated about these. This will call for medical courage.
Reporting to companies is important because companies are under a legal obligation to investigate the event and decide if their drug caused it. Companies are busy trying to get doctors and others to report to regulators who have no legal obligation to investigate and never link a drug to a problem.
Regulators never investigate because they strip away the names of doctors and patients and this immediately makes the reports worthless to us but gold-dust to industry.
Consider closing down the regulatory reporting systems run by FDA, EMA, MHRA and others – they are a waste of time. Some countries have pharmacovigilance systems that are independent of regulators and companies – strengthen these.

Companies are availing of an opportunity that opened up 420 years ago with the execution of Walter Raleigh. Raleigh was executed on the basis of testimony circulating about him outside of his trial. His detractors were not brought into court to be cross-examined. The injustice of this was recognized after his death and Hearsay was no longer accepted as evidence.
When regulators strip our names away – claiming confidentiality concerns – they transform our evidence into Hearsay or Anecdotes. But if our names remain on the reports and we and/or our doctors indicate a willingness to be cross-examined about what happened we may be able to help someone who is injured in the way we were or killed in the way one of our family was.
Encourage us, as the takers of a medicine, to report adverse events to companies and leave our names on the event. Remove our names and they are worthless to everyone except industry for whom this information is gold-dust – helping them to manage any debate about treatment hazards.
Without out names, we become Invisible and worse again add to the dangerous myth that RCTs give the best view of what drugs do – when even companies acknowledge that cross-examination does.
With our names present, we fracture a consensus that company propaganda aims at building about what drugs do and don’t do. Even Emperors can be put in their place by just one of us – even a young girl – speaking up.
Seventeen
Reform the coronial system, so coroners record in an accessible archive the drugs people are on at the time of death. This doesn’t happen now. After Age, when people die from Covid, vaccines or anything, the number of medicines they are is the greatest predictor of death but no one collects the details of the drugs they were on. Some are likely more dangerous with age or in medical emergencies than others.
Enable coroners to indicate that a prescription drug may have contributed materially to a death. They can’t at present. See Spotlight on the Suicides, The Coroner.
Advised by Medical Insurers, Doctors routinely betray us at Inquests. You will have to stop insurers advising doctors against stating that in their opinion a drug, prescribed in good faith, has killed their patient.
At inquests, at present, medical insurers de facto act in the interest of pharmaceutical companies rather than patients who have been killed by treatment.
You may have to limit the liability of doctors in order to embolden them to take a step that as a matter of honour they owe us but at present shirk. See Can Doctors Save Us.

Eighteen
The Access to Medicines campaign that secured access to Triple Therapy for HIV infections was one of our greatest ever moral and political triumphs within the health domain.
But current Access to Medicines campaigns increase polypharmacy and benefit industry rather than us. While reform of the patent system may help with this, it may not be the best option. An alternate approach would refuse to buy any new drug without access to the full trial data.
Universal HealthCare used to be a goal of progressive politics but it is rapidly coming to mean Universal Health Coverage, which for Pharma means that everyone has access to technical answers to health problems – as in the map above – and demand this as a right no matter what the cost. If Access to Medicine campaigns didn’t exist, Pharma would have had to invent them.
There is no meaningful Access to a Medicine without access to the data – a medicine is a chemical plus the information that informs its use. Having accessed the data on harms the public could be asked how much is this drug worth?
Industry pitch drug prices at what the market is prepared to pay for Sacraments – and we can be persuaded to build Cathedrals for Sacraments, while all around starve.
This would change if we are asked to weigh up How Much This Poison is Worth. Politicians are less likely to be viewed as rationing objects of desire to which we feel entitled if we are weighing up how much a Poison is worth.
Nineteen
In 1918, just after a Communist Revolution had been put down in Germany with Rosa Luxembourg shot, attempting to marry the Left and Right, Max Weber gave a lecture On Politics.
In it he said that government consists of political leaders and bureaucrats. The thing that distinguishes a Leader from a bureaucrat is that Leaders know there are times they have to act like Doctors and persuade their people to take their medicine. In Weber’s day this meant swallow a poison from which both the Leader and the People hope Good can be brought. He had no illusions that Leaders offer Sacraments.
For Weber a real Leader has a sophisticated mind in the sense that Scott Fitzgerald later defined this – a mind that has the ability to entertain two contradictory thoughts at the same time and still function. This is what doctors could once do – but not any longer. Entertaining two contradictory thoughts at the same time is likely to be something that focus groups can’t manage with quite the same reliability as they assess soundbites
Twenty
Where’s Greta?
Greta Thunberg’s generation stands as possibly the greatest symbol of the extent to which politicians, the media and many of us don’t get what is going wrong in healthcare. And if we don’t understand this, can we understand what is going wrong in the world?
Greta mobilized hundreds of thousands of her generation to come out and protest about the environment. A modern Joan of Arc.
But Greta’s generation is swallowing and demanding vastly more psychotropic and other drugs than any previous generation in history. Not for them any worry about becoming Stepford Wives or having their Revolutionary Consciousness dulled by Drugs.
They go to the Gym, eat their Veggie Burgers perhaps delivered by Uber, and see each other as empowered when they swallow Zoloft and Ritalin.
These drugs are now the second most commonly taken drugs by teenage girls after contraceptives. In the case of antidepressants like Zoloft, we have the greatest concentration of negative studies in human history – 45 out of 45 trials are negative – yet the consumption goes up and up.
In the case of these drugs, we also have the greatest known divide between what the academic literature says – the drugs work well and are safe – and what the data shows when accessed – the drugs don’t work and aren’t safe.
Any Leader who wants to save the World needs to do something about this. But this is not a moment for being seen to do things without understanding what is being done.
You have to avoid repeating JFK’s move on October 10 1962, which in response to the Thalidomide Crisis, effectively prioritized Efficacy over Safety, Technique over Judgment. Kennedy was a White Heat of Technology dude. Frances Kelsey kept Thalidomide – a drug proven to be Effective – off the US market on the basis of Safety. The judgment calls of people like Kelsey were undone that day in October.












‘only one – headache – ‘ …
‘Having accessed the data on harms the public could be asked how much is this drug worth?’
UK Parliament
GLAXOSMITHKLINE AND SEROXAT
Motion text
That this House condemns GlaxoSmithKline (GSK) for concealing for 15 years evidence that their anti-depressant drug Seroxat increases the risk of suicide and leads to `persistently worse’ depression; congratulates the US Food and Drug Administration for forcing GSK to confess that users of Seroxat `experience emergent suicidality or symptoms that might be precursors to worsening depression or suicidality’ and that `these symptoms may be severe and abrupt in onset’; and regrets the likely loss of life that has resulted from repeated denials of the lethal side effects of Seroxat by GSK, the Association of the British Pharmaceutical Industry and the Medicine and Healthcare Product Regulatory Agency in spite of the vigorous campaigns to reveal the truth by Professor David Healy, hon. Members, the Seroxat Users Group and Panorama.
Supporters (40)
https://edm.parliament.uk/early-day-motion/30692
Seroxat: Statement from GlaxoSmithKline
http://news.bbc.co.uk/1/hi/programmes/panorama/6291947.stm
In developing Seroxat, GSK has always been strongly conscious of the duty it owes to the millions of patients who suffer from depression and refutes any allegation that it has failed in this duty.
GSK utterly rejects any suggestion that it has improperly withheld drug trial information.
Depression is a severe and disabling condition and a well-recognised tragic outcome of the disease, particularly among young people, is suicide.
Careful monitoring of all patients is essential, regardless of whether they are taking medication or not.
GSK conducted nine studies over eight years to examine the use of Seroxat in treating children, those under the age of 18, with depression and other psychiatric disorders as treatment options for these vulnerable patients are extremely limited.
Results from these studies were documented and submitted to regulators in accordance with regulatory requirements.
No suicides were reported in any of the nine paediatric trials conducted by GSK and when reviewed individually none of these trials were considered by GSK or independent investigators to show a clinically meaningful increase in the rate of suicidal thinking or attempted suicide.
Only when all the data became available, at the end of the research programme, and were analysed together was an increased rate of suicidal thinking or attempted suicide revealed in those paediatric patients taking Seroxat.
GSK brought this analysis to the attention of the regulatory authorities, including in the UK.
Seroxat has never been approved by EU or US regulators as a medicine for those under 18 years of age and GSK’s UK product labelling has been entirely consistent with that position stating: “The use of Seroxat in children is not recommended, as safety and efficacy have not been established in this population.”
Disclosure of paediatric clinical trial results
From 1994 to 2002, nine paediatric trials were conducted in depression, obsessive compulsive disorder, and social anxiety disorder, involving over 1,000 patients treated with Seroxat.
The study results from the individual trials were provided to regulatory agencies worldwide in accordance with regulatory requirements. Upon completion of the individual studies, safety and efficacy data were also communicated publicly in a variety of formats such as peer-reviewed journals, and poster presentations at scientific meetings, as is common practice in the disclosure of data.
Study 329 was complete in late 1998 and was first submitted for journal publication in mid 1999. Full results of the trial were published in The Journal of the American Academy of Child and Adolescent Psychiatry in 2001. The study was also presented at several scientific meetings between 1998 to 2000.
Study 377 was also completed in late 1998. It was presented at the American Academy of Child and Adolescent Psychiatry meeting in 1999 and was referenced in a number of psychiatry journals from 2000. However, opportunities to publish these data in a scientific journal were limited as the study failed to establish efficacy for Seroxat.
Study 701 was completed in 2001. The data were presented as part of a combine publication at the scientific meeting NCDEU (New Clinical Drug Evaluation Unit) in 2002 and referenced in The Psychopharmacology Bulletin in 2003.
Analysis of data from paediatric clinical trial results
No suicides were reported in any of the nine paediatric trials conducted by GSK and none of these trials when reviewed individually by GSK or independent investigator showed a clinically meaningful increase in the rate of suicidal thinking or attempted suicide.
In the case of study 329 although a numerical difference in adverse events was observed by the company and the study’s investigators, for patients taking Seroxat compared to placebo, these findings were not, by themselves, considered clinically meaningful due to the limited number of patients involved and the fact that suicidal thinking and behaviour is a recognised symptom of the underlying disease.
Of the adverse events reported in this blinded study (where the patient, investigator and company do not know whether the patient received Seroxat or a dummy pill) only one – headache – was attributed by the independent investors to Seroxat. This opinion was published in the Journal of American Academy of Child and Adolescent Psychiatry in July 2001.
The subsequent paediatric studies – 377 and 710 – also were not considered to show any increased rate of suicidal thinking or attempted suicide.
Only when all the data became available at the end of the research programme and were analysed together was an increased rate revealed in those paediatric patients taking Seroxat. GSK brought this analysis to the attention of the regulatory authorities, including in the UK.
Your reference to “the possibility of obtaining a safety statement was considered but rejected” needs clarification. This showed that GSK had an honest belief that these preliminary data on safety were reassuring as the company was considering whether to submit new (more positive) safety wording for inclusion in the product’s label to regulators for approval. However, it was recognised that any submission could only be made in the context of efficacy as well as safety. Given the failure of Study 377 to demonstrate efficacy, it was rightly concluded that a submission for new wording on safety should not be made.
Promotion of Seroxat for treatment of paediatric depression
Seroxat has never been approved by EU or US regulators as a medicine for those under 18 years of age. Any decision to prescribe a medicine outside its authorised indications, in the EU or the US, is made by a doctor on the basis of his/her clinical judgement and the interests of their patient.
GSK does not promote its medicines for indications for which they are not approved and the company strongly refutes any suggestion that Seroxat was promoted to UK doctors for use outside the terms of the UK marketing authorisation, whether through clinical experts (KOLs) or any other route.
Internal documents prepared by GSK’s US subsidiary were specific to that subsidiary and were not distributed to the company’s UK subsidiary or market.
A memo sent to the company’s US sales force was produced to inform the sales force of the publication of study 329 in the Journal of American Academy of Child and Adolescent Psychiatry. As is clearly stated in the memo, this information was not to be used with, or distributed to, physicians, consistent with GSK’s global policy prohibiting off-label promotion. This memo was never issued or used by sales representatives outside of the United States, and would not have been seen in the UK.
The company did periodically receive direct unsolicited requests from doctors for medical information about use of Seroxat to treat children and adolescents with depression. As is standard practice, a ‘medical information letter’, was sent to the individual doctor. This letter identified published literature and clearly stated that Seroxat was not authorised for use in depression in patients under 18 years of age.
Can GSK Save the ….. ?
Supporters ( ) …
Exclusive Investigation (highlights refs to ‘surrogate endpoints’)
FDA allows drugs without proven clinical benefit to languish for years on accelerated pathway
BMJ 2021; 374 doi: https://doi.org/10.1136/bmj.n1898 (Published 30 July 2021)
What is surrogate endpoint in research?
A surrogate endpoint is a clinical trial endpoint used as a substitute for a direct measure of how a patient feels, functions, or survives. A surrogate endpoint does not measure the clinical benefit of primary interest in and of itself, but rather is expected to predict that clinical benefit.24 Jul 2018
I think One of the saddest consequences of the pandemic is the corrupt coercion of youngsters by offering incentives to accept vaccines The bribes in UK can amount to a hamburgher . Many have declined with rational reasons
The younger generations are not one cohesive cohort but include the well off with access to information and the income and knowledge of adults in their lives to back them in running campaigns and making decisions -and many on the periphery who cynically and often knowingly take the burgher. But it seems all groups are into prescribed drugs – but how many aren’t?
It would be interesting to know how many people are declining to take prescribed drugs opposed to those who do – and what the reasons would be across the board. That may be a hidden bit of optimism or lesson to be learned. ie whether there is evidence of any move away from accepting them. I know prescribing has increased overall but there may be a move away by some people in general not just through drug campaigning groups/orgs
I guess We can’t rely on doctors to seriously challenge prescribing when they have been indoctrinated during training and accepted the way medicine will be practiced . Otherwise why not drop out? Or do other aspects of practice outweigh the issue of lack of evidence/data ,loss of professional skills.
Where is the data kept? It must be somewhere. Who has the key? I just wonder if it was made available – what form would it take? Who would be trustworthy enough to present it ? And would it be comprehensive enough and understandable enough to all including to people who are prescribed medicines -so that it wouldn’t just depend on doctors reverting to doctor knows best if they did have access to the data? Instead of NICE knows best Data plus doctor knows best.
You refer to young people here Susanne and wonder if they will slowly start to refuse prescriptions for these drugs.
Shane and I are in the VERY early days of trying to set something up along these lines. We have another couple working with us so far. Our plan is to start with the fact that the environment / wildlife etc. are affected by chemicals in our ‘waste’ from the huge number of ADs etc. being used. We hope to get in touch with youth group leaders in our local areas to see if this kind of information would supplement work already being done on the environment. We’d hope that this could eventually cause youngsters to think along the ‘refusal’ lines. Obviously, getting group leaders to be onside will not be easy but it’s worth a try.
Alongside this we hope to meet with parent groups as we understand that many, in this immediate area, are at their wits’ end due to their youngsters’ anxiety issues. Our hope, in that area, is to share knowledge with such groups so that they at least know of the pitfalls that may face their children if they are prescribed these drugs. There is little that we can offer in the form of support, of course, but as we all here keep stating – LISTENING is such an important matter in itself and the fact that we, at least, understand their concerns.
Meeting with youngsters already on ADs is another thought but we have not yet worked through our thought on that particular section. I shall keep you posted!
So interesting and Such great work you are doing Mary – huge respect to you. and Shane. (In the meantime re the youngsters’ anxieties issues -from today as I exxpect you know -youngsters of 16 and over are being ‘offered’ vaccines without need for parental or ‘carers’ involvement. Just what is the age of consent in UK these days? – it’s a minefield. I can also imagine adolescents using this in the ‘wrong’ way to show they can ‘do what I like’ – common teenage behaviour. ) I would love to hear of how ‘things go’ . Best wishes
An Exceptional Article, it would be useful for all to read :
The Danger of Putting Youth on Antidepressants
Why medication shouldn’t be the default treatment for our kids’ mental health
BY PATRICIA PEARSON
ILLUSTRATION BY PETE RYAN
16:49, Apr. 5, 2020 | Published 11:15, Sep. 20, 2017
https://thewalrus.ca/the-number-of-youth-taking-antidepressants-is-growing-but-are-the-drugs-working/
I remember sitting in my Honda looking at the sample package, with Clara beside me, and noting the package’s black-box label, which warned of a heightened risk of suicidal thinking and behaviour in children, adolescents, and young adults.
“Why this drug?” I asked, “You don’t have major depressive disorder.”
“You’re not a doctor,” Clara said, with trademark teenage scorn.
Julie and her husband, Peter, now help run a website called rxisk.org,
Petitions
To -UK Government and Parliament -UK Citizens only can sign
Petition
Shift to a Wellbeing Economy: put the health of people and planet first
We urgently need the Government to prioritise the health and wellbeing of people and planet, by pursuing a Wellbeing Economy approach. To deliver a sustainable and equitable recovery, the Treasury should target social and environmental goals, rather than fixating on short-term profit and growth.
More detailsSign this petition
38,391 signatures
This response was given on 25 May 2021
While traditional economic measures such as GDP remain important, the government is committed to broadening the range of measures it uses, including by better accounting for natural and human capital.
Read the response in full
At 100,000 signatures, this petition will be considered for debate in Parliament
Share this petition
Facebook
Email
Twitter
Whatsapp
Created by
Laura Evelyn Sharples
Deadline
26 September 2021
All petitions run for 6 months
I think having anything to do with Wellbeing is a very bad idea. Its individual centred. And a rapidly growing industry where people eat veggie burgers, go to gyms and demand access to SSRIs
David
The name Wellbeing has the yuk factor but this petition doesn’t seem individually centred to me The petitioner has wider concerns So even though petitions rarely change anything, as the usual slick response from government shows here , , I think somebody who is using means to highlight issues effecting public health and the planet is probably not jumping on the individualistic wellbeing bandwagon. Petitions won’t do it but the support they get may encourage further action The Official Wellbeing lead in Wales by the way has no powers whatsoever to enforce action She can highlight issues and make them public but has no real political or critics point ,out much influence at all