
This image of Jorge Mario Bergoglio and Augusto Roux with Augusto as Obelix (of Asterix and Obelix fame), in the Roman Colliseum, will have to wait for a full explanation. It will make sense in Argentina and Europe but not in North America. A detailed explanation for North Americans will come next week. The posts are out of sequence owing to the interest that blew up about Fernando Polack in the last days.
So Long and Thanks for all the Fish is the fourth book of six in the Hitchhiker’s Guide to the Galaxy trilogy.
Funny Smell in City of Sweet-Smelling Aires
Recently, in Fishy Business in the Rio de la Plata, a bunch of us tried to shine a light on a notable Argentine medical entrepreneur, the amazing Fernando Polack, and the murky world of Buenos Aires politics, made murkier when you stir in vaccine politics. This world has Trump, Putin, Gates, Alfredo Fernandez, Jorge Mario Bergoglio (the pope) and Fernando mixing cheek by jowl.
If you’re still looking for a coven of Knights Templar, this might be a good spot to look.
An explosive tweet last Tuesday splattered murky water and fish from the Rio de la Plata far and wide. Lots of people have discovered Fernando and the fact that he recruited an amazing 4490 people to the Pfizer Comirnaty trial in 3 weeks. The question on many lips was how can one investigator do all this on his own? We can answer this one but there are other mysteries readers might be able to help with.

The two masked warriors, Fernando and Sergio, symbolically touch elbows in July 2020 after signing a mutual cooperation agreement for Polack’s trial to be held in Sergio Maldonado’s Hospital Militar Centro.
Fernando didn’t do all this on his own. He and his gang (see Fishy Business) run an SMO and had 467 doctors around Buenos Aires linked into the operation. They had been running other trials for several years, heavily funded by Gates and other players.
There is a Fernando operation in Panama, perhaps just an empty office linked to money-laundering but who knows. We are way out of our depth here and need investigative journalists to join in.
His SMO company i-Trials was opening up branches in Florida earlier this year but coincident with our “Fishy Business” blog, the iTRIALS website disappeared and reverted to being “Under Construction”. The original version can be seen Here – complete with imported clip art.
Letter to Reviewers
When the Eric Rubin Boston Strangler post ran, followed by Fishy Business, we fished out the names and contact details for all New England J of Misinformation peer reviewers for the first half of 2021 – roughly 350 people – and emailed them as follows:
This email is being sent to all living reviewers listed by NEJM for the first half of 2021. It will also be sent to 140 participants on a listserve interested in pharma-related matters, who are close in profile to the NEJM reviewers.
Attached is a letter I have sent to Dr Carol Allen, President of the Massachusetts Medical Society, who are responsible for the journal, outlining general concerns made more specific in the links below.
My general concerns center on a lack of access to clinical trial data for all drugs and the ghostwriting of the clinical trial literature. The Pfizer vaccine has made many people aware that there is no access to the data from trials or that access might take decades. The published NEJM trials also make it clear that medical writers wrote the articles.
The phrase fraud on the court in the letter to Dr Allen is used imprecisely. Strictly speaking it would refer to a lawyer commissioning a study known to be misleading in order to defend a case in court. We are not however far removed from this scenario.
Fishy Business in the Rio de la Plata
I hope you will not be put off by the Lurid title and opening image in the first link. This is driven by the critical pass we have reached and the tone of some NEJM responses on these issues.
I try to ensure posts are fact-checked, but mistakes on issues as complex as these can slip through. I will correct anything pointed out that is obviously wrong.
I invite you to email any comments to me on any aspect of this material. You can also comment publicly on one or other of these postings and if indicated I can ensure your name and contact details do not appear.
Anonymous or not, no linkage will be made by me between your name and any comment you send unless you make it clear you are happy for a link to be made.
I have no plans to publish any comments. At most I may offer an indication of the rate of response and offer an aggregate assessment of the tenor or tenors of comments.
You can also contact me independently of this exercise David Healy
No reviewers disputed that most of the literature on vaccines and drugs is ghostwritten. It would be difficult to do so when the NEJM vaccine trial articles state clearly they are ghostwritten.
No reviewers disputed the claim there is no access to the trial data. It would be difficult to do so given the world knows Pfizer didn’t want to release anything for 75 years and Stephen Thomas, lead influencer in this trial, has made it clear he has not seen the raw data.
No reviewers have made contact, bar one wondering when the fraud on the court article will appear.
Elizabeth Loder and Michelle Mello both of whom have an interest in clinical trial data – the responsible access version – didn’t respond.
A Fernando auto-response said he was on sabbatical.
Guardian Angels
A fascinating wrinkle has now appeared on Snow White’s face and seems to be extending. Do any of the 350 dwarves gathered around her glass casket have an inkling about how the story goes from here?
In Fishy Business, we were under the impression there was one mega site in Buenos Aires, Fernando’s. The NEJM article on which he is first author gives the impression that he recruited an astonishing 5,764 patients.
Except some of the dumped Pfizer papers now show he actually only recruited 4490 at his 1231 site. This was in 3 weeks – so 1500 per week or 20 per hour in a 10-hour day. With a network like Fernando’s, this is doable. Augusto Roux, who was there, however, says it was chaotic and he was nearly given two vaccines on the same day.
While all this frantic activity was going on in August 2021, Fernando found time to set up a new enterprise in Buenos Aires – Gana(te) La Vida S.A. (Get a Life) with his son, Leandro, to provide gastronomic delights for pubs, clubs and bars with a capital of $100,000.
All this has been ‘out there’ for a while. Now though a new site 4444 has swum into view, as spotted by @Jikkyleaks on May 9 2022.
Angel Numbers can be associated with many spiritual attributes. For instance, “The angel number 4444 is associated with difficulties. It is difficult for us to accept the fact that we will face hardships in life, but our Guardian Angels are here for support and guidance in overcoming these obstacles.”
Is site 4444 a Guardian Angel? Like Guardian Angels, it does not exist according to the site data so far released. The sites are numbered consecutively from 1001 to 1270. We need to explain 270 – given there were only 152 or 153 sites for phase 2/3. Have phase 1 trial sites also been listed here? See Comprehensive (sic) List of All (sic) Clinical Sites
Ghost Site 4444 reeled in 1274 volunteers in just 5 days in late September 2020. Why the rush?
Of these volunteers ninety-nine per cent are coded white “Hispanic/Latino”. Reported adverse events from the ghost site come from subjects in Argentina.
And if you add 1,274 to 4,490 you get 5,764 which is exactly the number recruited from Argentina reported in NEJM – more exact, that is, than almost anything else reported in this trial.
And the beautiful Alejandra Herrera Jure, an Argentine ophthalmologist, who now works for Pfizer, says she worked at both 1231 and 4444 sites.
There were 466 doctors other than Alejandra listed in the data dump – someone reading this must know one of them who can shed light on what’s up. They are listed HERE in an 81 page document, their names taking up pages 58 to 71
Why this extraordinary effort to chuck another 1274 volunteers into the mix, three weeks after normal recruitment had stopped, just before data closure in the trial? Was this a Special Military Operation, also run from the Hospital Militar?
It may be innocent. A lot of Media Hype in July 2021 in Argentina resulted in:
“Just two days ago the call for trials of the coronavirus vaccine was opened and there are already more than 15,000 volunteers who requested to be part of the experiment. The figure exceeds the amount required for Phase 3 of the clinical trial to be carried out in Argentina.” [28 Jul 2020]
Perhaps thinking that being part of the trial might protect them from Covid, as Augusto thought, they were falling over themselves to get in the trial.
Maybe after the 1231 caravan rolled on Pfizer said to Polack “hey we could do with a few more subjects” or Polack said to Pfizer “hey I’ve got another couple of thousand begging to be in the trial” and they made a deal for a rush job in a new trial centre 4444.
At the moment, we have a mystery to rival Pfizer’s B2 or not B2 agonies – as related by Alberto Moonshot – over whether to proceed with BNT162b1, on which they had lots of data or jettison all this data in favour of a slightly different BNT162b2. Astonishingly they dumped b1 and ran with b2. We still don’t know why.
Augusto Contra Mundum
Fernando is fascinating but we have been more concerned with and for Augusto Roux, who volunteered for the trial and was clearly injured but who ended up being told no he wasn’t – he had Covid even though the test was negative. And then not just told he was mad but had madness recorded in his medical notes diagnosed by Fernando – a pediatrician and infectologist. – is there no end to the man’s skills?
On April 6, I wrote to Peter Marks of FDA and to the Argentine regulatory authority ANMAT as follows:
Dear Dr Marks
I refer to the report by Ramachandra Naik, PhD, Review Committee Chair, DVRPA/OVRR on the COVID-19 Vaccine, mRNA, Proprietary Name: COMIRNATY
Section 7. “Safety and Pharmacovigilance” pages 23-24 under Myocarditis/Pericarditis states
“During the time from Dose 1 to unblinding in Study C4591001, one report of pericarditis was identified in the COMIRNATY group, occurring in a male participant ≥55 years of age, with no medical history, 28 days after Dose 2; the event was assessed by the investigator as not related to the study intervention and was ongoing at the time of the data cutoff. One report of myocarditis was identified in a male participant <55 years of age in the placebo group, occurring 5 days after his second placebo dose.”
I wish to report one further incident of confirmed pericarditis in a participant in this trial.
Augusto Roux, male, aged 36, study number 12312982, was enrolled on 21 August 2020 into trial centre 1231 in Buenos Aires, Argentina, Principal Investigator Fernando Pedro Polack MD.
He received two injections as part of the trial on 21 August 2020 and 9 September 2020. Both injections were later shown to have contained the active vaccine BNT162b2 (30 μg).
After his second injection, he became unwell over three days with a fever and vomiting. On 12 September, he was admitted as an emergency to Hospital Alemán in Buenos Aires, ref 677259. Investigations for Covid-19 infection were negative. A CAT scan of the thorax performed on 13 September was reported as showing a laminar pericardial effusion. His illness was diagnosed as an adverse effect from vaccination. He was discharged on 14 September.
He made a slow recovery. The pericardial effusion spontaneously resolved.
Four doctors including myself, two with considerable pharmacovigilance expertise, have examined Mr Roux and both his medical and clinical trial record. We are of the view that he clearly had a pericardial effusion, most probably due to a pericarditis and that this was triggered by his vaccination with Comirnaty.
It is not within the remit of Dr Naik’s report to discuss why this adverse event was not reported to the FDA by the sponsors of the trial. As it stands, however, Dr Naik’s report is incorrect, and this should be remedied.
Mr Roux reported his injuries to ANMAT (National Administration of Drugs, Foods and Medical Devices), the Argentine equivalent of FDA. ANMAT held a hearing concerning his case on October 8. There was input to this hearing from Pfizer. The Buenos Aires site was the only site in the study that had both a routine audit, as well as a non-routine audit, the latter in the week of October 13th to 20th, conducted by a Pfizer employee, Andrea Mohr, shortly after the ANMAT hearing into Mr. Roux’s case.
Given the trial records and clinical records I have, and the ANMAT hearing information, it seems clear that Mr Roux received the active vaccine in the trial, and was injured, but has since disappeared from it.
Is he one of the 311 participants who were excluded because of an important protocol deviation after dose 2? Have FDA been informed of just why these 311 were excluded and have FDA had a chance to decide whether events such as a pericarditis in this case should have been included in the final representations to them even though the subject was excluded for efficacy purposes?
Mr Roux has stated that he would be willing to supply FDA with further information, including his clinical and trial records, in order that the true facts are acknowledged and recorded.
I would be grateful for whatever light you can shed on this situation.
Yours sincerely David Healy
In the letter to ANMAT I made it clear I did not want to be saying anything incorrect about this case and so would welcome any other detail they could provide. There was an email auto-acknowledgement from FDA, and nothing from ANMAT.

The image above is from a Pfizer trial on a new RSV vaccine. It is heavily redacted as is typical for the documents being released by Pfizer. Fernando Polack is a leading RSV expert.
The document below is from ANMAT’s hearing on Augusto’s complaint about how his case in the Pfizer trial has been handled.
Which of them shows evidence of Dr Polack?

The second one. The Polack signature is second from the left on the top line.
Ave Jorge
Argentine Society is tightly interwoven. Many of the key people know Augusto and he them. This includes Jorge Mario, now based in Rome. Hence the image above. If Augusto had never met Jorge, he might have said Ave Abba, which for other reasons might be a good way for Fernando to address Jorge or perhaps Jorge, if he has a sense of humor, to address Fernando.
Jorge does not have a track record in helping the Disappeared from Drugs or Vaccines. This is odd given his mission to remind the faithful that the cornerstone the builders rejected is the cornerstone the mission of the faithful is built upon.
I wrote to Jorge about these matters 6 months ago not mentioning Augusto by name but with him in mind. See Healy to Bergoglio. There was no response.
Perhaps having Augusto clearly named and in the frame or mention of an UFA or UFF will spark Jorge’s interest – Unidentified Flying guardian Angel or Unidentified Flying Fish?
Data Dumps
Let’s have a closer look at some of the other data from Polack’s Hospital Militar Central site in Buenos Aires.
You would expect the distribution of something like Height to follow a normal distribution curve, especially when there are thousands of data points. The data here come from a PHMPT document that is over 3000 pages long.
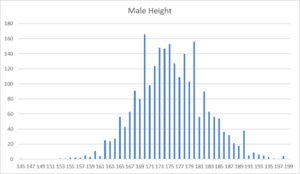
Not in Fernando’s trial. There are twice as many men 170cm tall compared to 169 or 171cm. This means the subjects have not had their heights measured, as would have been required by the protocol.
Just plotting out basic data like this is one of the ways to detect fraud. In this case, the fraud may have a simple explanation and may be excusable. People were asked their heights and volunteered 170 rather than 169 or 171. The same happens at the 180 point. It shows actual measurements were not taken or were hurried – but so what.
The cornerstone data below from Jikkyleaks are more interesting than ours. In this case, adverse events are plotted and you’d expect the scatter of data points that the rest of the graph shows – except for Germany and Turkey.
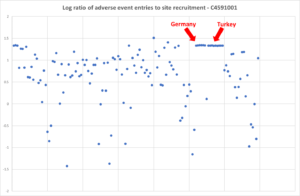
There is a tradition in Germany of only having the adverse events you are told you can have but still this is extreme. Whatever about Germany, you’d expect Turkey to be more sensible.
Suny Side Up
Fernando’s co-joint author on these papers was Dr Stephen Thomas of SUNY Upstate Medical University and fellow at the Trudeau Institute in Saranac Lake, where he is a big fish in the world of infectious diseases.
In the last millennium he would have been called a KOL – key opinion leader. An “Influencer” in todays parlance. So much so that he was hauled in as lead researcher to coordinate the Pfizer COVID-19 vaccine trial and several other antiviral drug developments.
Egg on his face? Suny side up
In an interview this is how he describes his role:
Q: In terms of communication, how do you go about coordinating teams and information globally?
A: My role as the coordinating principal investigator for the Pfizer-BioNtech vaccine trial is not an operational position. Meaning, I’m not actually coordinating the different sites participating in the trial, this is accomplished by Pfizer and Pfizer’s partners. My role, in addition to leading the Upstate site, is to be an external reviewer of the data, which will be submitted to regulatory agencies for consideration of emergency use authorization or licensure. Coordinating principal investigators provide a fresh set of eyes and help to identify any potential questions about the data or how to interpret the data.
We wrote a nice letter to Dr Thomas, as principal investigator for Pfizer’s trial and joint lead author of an NEJM paper on the safety and efficacy of their vaccine – although he claimed not to have access to the original data. The two points we raised were first, the discrepancy in the number of dropouts from the trial after the second vaccination. According to the chart published as “Figure 1. Screening, Randomization, and Follow-up”, 11 subjects in the active group and 24 in the placebo groups dropped out, whereas the recently released FDA data stated that comparable numbers were 311 in the BNT162b2 group and 60 in the placebo group.
Our other query was to see whether there was an explanation why, having trawled through the data, there were no recorded instances of pericarditis documented, despite the clear evidence that volunteer number 12312982 from Argentina was diagnosed with this condition 3 days after his second injection of the Pfizer trial vaccine.
(We didn’t mention that Fernando, a co author, knew all about this adverse event).
Dr Thomas has not responded. He is well placed to know something about 4444 – which as we’ve said above may have an innocent explanation. Maybe he’s doing the Guardian Angel Tango. There is one. This is the most respectable image:

There is also a Tango 44 option. And there is this fabulous 1904 image of men doing the tango in the Rio de la Plata.





“One report of myocarditis was identified in a male participant <55 years of age in the placebo group, occurring 5 days after his second placebo dose.”"
What a joke, there are cases of myocarditis everywhere the vaccine is given, the risk is 10 times higher in the week following the vaccination (CDC data computed from an SCRI and shown at the ACIP meeting of June 2021) but they say this "male participant <55 yo" received the placebo ?
“Augusto is not a conspiracy… He volunteered because he felt it was his best way of contributing:
https://vaccineharm.wordpress.com/2020/12/29/augusto-german-roux/
“Do you realize the implications of this last comment?”
Fernando Polack, with Levy Yeyati: “We did not have the early Pfizer vaccine due to a series of errors”
https://www.zyri.net/2022/03/17/fernando-polack-with-levy-yeyati-we-did-not-have-the-early-pfizer-vaccine-due-to-a-series-of-errors/
-Diversify, fill the board, you have to put a chip on each number of the roulette because it’s still cheap. Even if you lose all 35 chips and win with one. That was the mistake, it seems to me, that there certainly was: a poor understanding of the party that was on the table at the time.. Seeing it from where I saw it… but I was in one of the vaccine safety commissions, at the American, European level, I was everywhere. I knew that you had to play everything, I knew that you had to be aggressive in everything.
Yes, I was a mess. What about the Pfizer study? If there was a quarantine and Pfizer couldn’t come to work here. They were actually much more invested in our expertise than they normally would have been in a normal situation. There was all the political attention that there was in Argentina, around all this controversy, where I did not participate, but I saw it constantly, and sometimes I even heard someone who named me.
And there was all my responsibility, which is much more important to me, which is how the patients were going to fare, how the volunteers were going to fare. If they had a fever, if they felt fine, if they were contained. 5,700 people, 26 times more than the average for a North American site. We had 26 times more people who came out perfect in the study than the average center from Columbia, or Harvard or Stanford, for example.
We were exposed to death, let’s not fuck around,
What happens is that Pfizer has a philosophy that seems very attractive to me, and that is that we work, we do not report our work. That premise governed my entire team and therefore governed all the progress here. That’s why you didn’t see me anymore. I dedicated myself to the success of this study, to the care of the volunteers, to the evaluation of the vaccine. It’s not that I get rich if the vaccine works.
Because science is the growth of peoples …
One of the interesting things about this comment is the idea that Columbia, Harvard or Stanford were putting people into this trial. They weren’t. It was Hialeah in Florida, Ventavia in Fort Worth and Houston, and outfits in Nebraska who were entering patients along with some operations in Brazil.
D
‘
Above ‘ Elizabeth Loder and Michelle Mello both of whom have an interest in clinical trial data – the responsible access version – didn’t respond.’-
In 2015 E Loder said:-
Why Medical Journals Must Make Researchers Share Data From Clinical Trials
The Conversation
July 6, 2015
Lab results. (Credit: Shutterstock)
This month a new BMJ policy on sharing data from clinical trials takes effect. From July 1 2015, the authors of all clinical trials published by the journal must agree to make individual patient data from the trial available to other researchers upon reasonable request. Among major medical journals, only PLOS also requires data sharing as a condition of publication.
There are many advocates for data sharing, including the National Institutes of Health, The Cochrane Collaboration and the World Health Organisation. A 2011 statement from the Cochrane Collaboration, for example, called for sharing:
All data from all randomised clinical trials, including raw anonymised individual participant data that do not allow identification of individual participants, and the corresponding trial protocols to become publicly available free of charge and in easily accessible electronic formats.
A landmark report released this April by the Institute of Medicine concluded that data sharing is in the public interest and should become “the norm”.
But sharing is as yet not standard in the medical research community. Instead, the current research enterprise is probably better characterised as a “walled garden” in which most industry and academic investigators carefully control access to the information collected in clinical trials.
This hoarding of information has many bad consequences. It prevents other researchers from checking the accuracy of any results the original researchers might have published. It also makes it impossible to tell if those researchers have manipulated data or selectively published information about the study, which are problems that continue to occur. Most importantly, data hoarding prevents other scientists or patients from using clinical trial information to inform or accelerate their own research.
Hoarding or selectively publishing data. (Credit: Shutterstock)
The the problems caused by making research data inaccessible are not mere abstractions: they affect real people. One widely mentioned example is the case of the Italian scientist Alessandro Liberati, who was unable to obtain information from clinical trials that were relevant to decisions he had to make about how to treat his own cancer. Among the evidence pertinent to his decision were “four randomised controlled trials, whose results haven’t yet been fully published …”
In an opinion piece describing his plight he asked “why was I forced to make my decision knowing that information was somewhere but not available?” Liberati concluded by saying that “research results should be easily accessible to people who need to make decisions about their own health”.
Clinical trial data should be thought of as a public good. This has been defined as:
a good that does not diminish in value with use, and has (almost) no marginal cost for users after the first use. A scientific public good is special because its value increases with use and it is important to maximise us of the information obtained from the sacrifices of human participants.
Sharing data will accelerate medical progress because discovery is a cumulative process that builds on previous work. If that previous work is not available then scientific progress suffers. Harlan Krumholz, Yale researcher and open data advocate, has written that “many trials yield only a fraction of the knowledge that could be produced with more resources and creativity.”
There is already a degree of collaboration among scientists and research groups around the world, but imagine how much more productive it might be if rivalries were dissolved by freeing up information. For one thing, sharing of clinical trial data would make possible creative research outside the current set of stakeholders and scientific insiders.
An additional benefit of wider sharing of data from clinical trials is that it might help restore trust in the clinical research enterprise. At present, according to the Center for Information and Study on Clinical Research Participation: “Public sentiment toward the clinical research enterprise is at an all time low … more than 70% of Americans believe that drug companies put profits ahead of patient needs.”
It is important to counter cynicism about medical research. If patients and doctors lack confidence in medical evidence, what hope is there for evidence-based medicine? If the evidence on which guidelines and recommendations are based is not trusted, how likely is it that doctors will apply therapies or act in other ways that are consistent with the best evidence?
Despite all of the benefits we can expect as a result of increased sharing of research data, there will of course be problems. It is inevitable that serious unanticipated consequences will occur. But even knowing that, it is still the right thing to do. That is because there will also be many unanticipated serendipitous and important benefits as well. The balance of benefits and harms is almost certain to be favourable. We should have confidence that human and scientific ingenuity will be able to solve the problems that emerge.
We are engaged in one of the great struggles of human knowledge — the struggle to liberate clinical trial data and ensure it is put to its highest and best use, now and in the future. Medical journals are in a position to help make data sharing the norm by requiring it as a condition of publication. The BMJ calls upon other journals to help make this happen.
The Conversation
Elizabeth Loder is BMJ acting head of research and associate professor of neurology at Harvard Medical School.
This is delicious in that Dr Loder appeared to be actively trying to stop Study 329 and the sharing of GSK’s trial data at just this point in time. She was then actively involved in what appears now to be an industry effort to control access to data while appearing to endorse access and hoodwinking lots of people including it appears to me the NEJM and BMJ.
Whether she herself understood any of this is the key point
David Healy
David, great article and kudos for all of your amazing investigative work. So many threads to pull together. In the raw data released via FOIA, it is clear that the ID numbers beginning with 4444 are attached to site 1231 (the Argentina site), even though their ID numbers start with 4444. Their recruiting was done in late Sept., and I expect your explanation here is correct: Pfizer realized they needed more recruits in short order and turned to the Argentina site to help fill their order.
One interesting thing to note in the data: Augusto Roux’s unblinding date is missing, though we do know he was unblinded. There are about 500 more subjects in the treatment arm who are missing unblinding dates compared to the placebo (who have ~2000 people who did not unblind). Why would that be? One hypothesis is that they knew they had received the real vaccine (either b/c of the reactogenicity or b/c they were given paracetamol prior to receiving the injection), and so didn’t feel a need to unblind when giving the chance. (Under the assumption that a missing unblinding date means they were never unblinded.)
Another hypothesis is that they (or some of them) were in a situation like Roux: they demanded to be unblinded because of a severe AE, so their unblinding date went missing…
Josh
Thanks for this. These are exactly the kinds of extra details and thinking about the details that is needed
David
So Long – and Thanks for all the Fish …
As people being digging into the detailed records of the Pfizer/BioNTech COVID-19 vaccine trial released by the FDA, there is something they need to understand about how the trial was run.
https://jackanapes.substack.com/p/the-pfizer-vaccine-trial-was-not?utm_source=%2Fprofile%2F42568025-josh-guetzkow&utm_medium=reader2&s=r
Yes, the protocol makes all kinds of promises about how this unblinded analysis team will be insulated from the other researchers and their reason for being is just to share data with the unblinded data monitoring committee (DMC). But it would be naïve in the extreme to take the word of perhaps the world’s most prolific corporate career criminal, Pfizer, especially since we saw the way they’ve misreported and tried to cover-up 12-year-old Maddie de Garay’s severe reaction to the vaccine.
One excuse given for this flagrant violation of double-blind studies seems to be that since the trial had to be done at “warp speed,” they needed to analyze the data simultaneously. They couldn’t wait until after the study was completed. This contradicts what we’re told, which is that no corners were cut even though they developed and tested a vaccine in record time. (Of course studies like this have unblinded DMC’s for safety monitoring during the study, that is common practice, but the extent of unblinding here is not.)
Here they discuss how the “unblinded statistical team” would perform interim analyses at various points throughout the trial:
A possible explanation is this
Augusto WITHDREW from the study on 23 Sep 2020
He was UNBLINDED on 9 Oct 2020
Therefore he was not in the study when unblinded
March 2:
The clinical safety data for the Pfizer trial states 302 people on the active vaccine dropped out for protocol violations. A recently released document giving dropouts lists over 200 dropouts for the Buenos Aires site – but this does not include Augusto. This appears to mean Augusto is registered with Pfizer and FDA as a case of Covid within the 7 days of dose 2.
https://davidhealy.org/disappeared-in-argentina/
There is a gap for ten months in the event record. Then on August 12, 2021, after Augusto had taken his complaints to FDA and others, a Dr. Julieta Gelardi (an ophthalmologist – Don’t Cry for me Argentina) made this entry:
I hereby clarify that the evolution of Adverse Event 2 “Severe Anxiety” on 21/OCT/2020 of Dr.María Alejandra Neira, and from 30/OCT/2020 of Dr Fernando Polack did not need to be loaded up to the CRF since the volunteer withdrew his consent to continue participating in the study on 23/SEP/2020, at which time the event was in progress and its status was unresolved, unrecovered. As mentioned in the Notes of V2 by Dr Polack the former volunteer gave authorization to continue monitoring in the context of security monitoring, which is why updates were made to the digital medical record.
The CRF (Clinical Record Form) is the material that went back to Icon, the Dublin-based company running the trial, analyzing the data and ghostwriting the New England Journal of Medicine articles, the first of which ends up with Dr Polack as its first author. So neither Icon, nor FDA, nor the NEJM stand much chance of finding out that there has been an event they should ask Dr Polack more about.
“As mentioned in the Notes of V2 by Dr Polack the former volunteer gave authorization to continue monitoring in the context of security monitoring, which is why updates were made to the digital medical record.”
‘We can imagine other types of fraud: a person’s adverse event is minimized or re-classified if it is known they received the vaccine, and/or the opposite if it is known they are in the placebo group. The sky’s the limit, really.’ – Jackanapes Junction …
Another ABBA classic ‘Angel Eyes’
‘Look into his angel eyes, one look and you’ re hypnotised… You think you’re in paradise….. But he comes in disguise….l
Is Subject #12312982 the Key to Proving Pfizer Vaccine Trial Fraud?
The Story of Augusto Roux
Josh Guetzkow
Subject # 12312982 in Pfizer study C4591001 is Augusto Roux, a 35-year old lawyer from Buenos Aires, Argentina who volunteered for Pfizer’s stage 3 trial of its COVID-19 vaccine (or whatever you want to call it) in order to protect his mother with emphysema.
His story and some of the shenanigans surrounding the Argentinian trial site have been amply covered by Dr. David Healy in three sprawling but extremely important blog posts: 1, 2, 3. The first one was published March 1st, but it was only last week that I caught on to this story, so I’m assuming most of you probably aren’t familiar with it. So please share this — we’ve got to get the word out, because Augusto Roux may very well hold the key to bringing down the Pfizer vaccine trial, or a least proving fraud at the largest trial site that was home to over 10% of the participants in the trial.
If you don’t have time to sink your teeth into David’s posts (even just the 1st one), I’ll bring you up to speed on key details:
https://jackanapes.substack.com/p/is-subject-12312982-the-key-to-proving?s=r
Now here’s where it gets really interesting, and we know all of this because Augusto, a lawyer, successfully sued to get his medical and trial clinical records, even though it took him over a year. I will try to make a long story short:
There are many fishy things in the protocol deviation data, which I will be writing about soon. So, as they say, watch this space!
Josh Guetzkow
@joshg99
Interview with Augusto Roux
https://www.twitch.tv/gigaohmbiological
Starts around 19..
It would be stupendous if David Healy’s exposure could reach more of those who are being targeted presently as candidates for a 4th vaccination. The publicity has varied from targeting the over 50s to over 80s. as well as several vulnerable groups ,members of which have reported harms many , most ,of which are not reported (Yellow Card – what’s that?)
FDA NEWS RELEASE
The safety of Moderna COVID-19 Vaccine, when administered as a second booster dose, is informed by experience with the Pfizer-BioNTech COVID-19 Vaccine and safety information reported from an independently conducted study in which the Moderna COVID-19 Vaccine was administered as a second booster dose to 120 participants 18 years of age and older who had received a two-dose primary series and a first booster dose of Pfizer-BioNTech COVID-19 Vaccine at least 4 months prior.
No new safety concerns were reported during up to three weeks of follow up after the second booster dose. (Call me Pinochio)
By Helen Johnson
Friday, 20th May 2022, 3:36 pm
the NHS recommends having the fourth dose about six months later 9than the 3rd). (or 4mths according to some expert advice or take your choice it seems, people are not tested first to see if they need a booster)
The Joint Committee on Vaccination and Immunisation (JCVI) has advised the Government to provide an autumn Covid-19 booster programme in preparation for winter.
“We have provided interim advice on an autumn booster programme for 2022 so that the NHS and care homes are able to start the necessary operational planning, (0n the basis of this ‘research’)
“As we continue to review the scientific data, further updates to this advice will follow.” (The ‘updates’ will be too late obviously)
A UK trial recently found that a fourth dose of a Covid-19 booster vaccine increases protection against coronavirus, particularly in those aged over 70,.
The study was also small so more research is needed over a longer period of time in order to track how long the immune response lasts
News
Covid-19: Pfizer asks US regulator to authorise fourth vaccine dose for over 65s
BMJ 2022; 376 doi: https://doi.org/10.1136/bmj.o711 (Published 17 March 2022)
Cite this as: BMJ 2022;376:o711
Look under any stone we find corruption and misinformation –
MONEYPOX
We’ll need to keep an eye on WHO gets their dirty hands on the monkey pox vaccine. Seems their might be a bit of pocket money there
, May 21, 2022
Monkeypox: The only company that manufactures the vaccine for this disease soars on the stock market
In just two days, the Danish company Bavarian Nordic managed to raise its shares by up to 53% on the stock market, as the biotech company is the manufacturer of a smallpox vaccine. Yesterday Bavarian Nordic’s shares rose 25 percent, allowing it to end the day with gains of up to 18 percent, according to El País.
Last Thursday, the company announced that the United States will pursue a $115 million contract option to purchase this version of the vaccine from Bavarian Nordic.
Also on Thursday, an announcement was made by the pharmaceutical company that it had secured a contract with an undisclosed (why?)country in Europe to supply the Imnavex vaccine due to an increase in cases in May this year.’ (Any ‘research’ on the interaction of Covid vaxxes with Immavex?). Hopefully gay rights activists might jump in on this , as they did so admirably when the disgusting AIDS scares of mainly the 80s motivated massive public campaigns)
Argentina wakes up to the Polack problem
25/05/2022 Radio Rivadavia am 630
[Here Claudio Zin a doctor and politician is interviewed about adverse effects from vaccine trials – in particular Pfizer’s Argentine trial. Translated from Spanish]
Dr. Claudio Zin: Let me tell you that there is a sinister plot behind this story, because in many cases of side effects during the research protocol, many cases, the side effects were not reported, so the Pfizer vaccine came out of the research protocols unscathed, phase III research protocols in Argentina for example, and now there is an in depth investigation in the world of science about whether there were as many cases as suspected, if these cases were not reported as suspected, and if that lack of reports involves someone who is a promoter of the Pfizer vaccine, Polack for example. There is an ongoing investigation on a couple of cases of side effects that were intentionally not reported, I’ve been behind this issue for many months, you know, I once commented on it, but there will be some sinister revelations about the Pfizer vaccine.
Woman: now Doc, in the United States, in the rest of the world where this vaccine that has 95 percent efficiency, it has been proven to be the best vaccine, of course.
Dr. Claudio Zin: absolutely, without a doubt, without a doubt, but that does not mean that he does not report – he has a moral, legal and ethical obligation to report adverse effects.
Woman: in the midst of the pandemic, and in the midst of the studies, which were, which speeded the development…
Dr. Claudio Zin: It is true.
Woman: Surely we are going to see things that were, that were missed because there was an emergency situation with that and other vaccines, right?
Dr. Claudio Zin: true, but I would have been wary about the Pfizer vaccine if I knew whether there were 100 cases of pericarditis.
Woman: Yes me too, in fact I had it myself.
Dr. Claudio Zin: but there are things from the point… obviously, there are facts that from the ethical, moral point of view and of all the treaties that concern the protection of the patient, one has to carry out, as a doctor and as a researcher, regardless of frightening or not frightening, saying or not saying, this is out… the scientific method is out of a context of temporal convenience, I cannot adapt the research protocol and its results to the fact that people need this or that thing, it doesn’t work like that, if someone understood that it works like that, they’re wrong.
Woman: Suppose I wanted to consult you, what does a doctor have to do? I go to a doctor and tell him that I have certain symptoms, does he do all the check-ups and really check, what are those steps?
Dr. Claudio Zin: Make a complaint, there are some forms, for instance, you go to the ANMAT website, there is a special place for doctors, under the section called pharmacovigilance where you can notify a probable adverse effect of the product, and then ANMAT is in charge of investigating whether it is, or is not.
Woman: So it is ANMAT, not the pharmaceutical company.
Dr. Claudio Zin: No, ANMAT, our benchmark in this type of thing is ANMAT, it is ANMAT that approves the protocols, ANMAT that registers the adverse effects, and ANMAT that investigates and classifies each adverse effect that is notified, and decides whether it can be linked or not, or possibly linked.
The interviewer is Cristina Perez. Here is a recording of the interview, in Spanish – https://www.youtube.com/watch?v=j_8SNPd1uWY
Great article!