
As of today, the BMJ have come on board with the contents of this post.
There is an article by Hristio Boytchev – see Maternal RSV Vaccines
And an editorial by Elizabeth Robinson and Rosalind Smith – Preventing RSV.
Britain’s Daily Mail, possible the best newspaper for coverage of health issues, is also now covering RSV Vaccines. It will be a long time before the Guardian ever gets round to doing so.
From our Immunological Correspondent
A Shot in the Dark (1964) was the second movie in the Pink Panther series which launched Peter Sellers’s career as the bumbling French detective, Inspector Clouseau.
By a strange quirk of fate, between February 22-24, 2023, two pivotal meetings to determine the future of RSV vaccines in pregnancy were held.
In Atlanta, Georgia, ACIP was debating the future of vaccines for RSV, with a view to advising the FDA on whether they should be licensed.
Meanwhile, 4,000 miles away in Lisbon, Portugal, the annual RSVVW meeting was being held at the lush five-star EPIC SANA Hotel. Conference fees were up to € 1065 – not including accommodation and travel. What would Greta say?
RSVVW? It is the annual conference for ReSVinet, the Dutch-based, industry sponsored, Respiratory Syncytial Virus Foundation. Not many of the 550 participants from 48 countries would be aware that the initials RSVVW disclose its aim: Respiratory Syncytial Virus – Vaccines for the World.
The Race
The background to this was the race to be first past the post with a RSV vaccine that would be given to every pregnant person in the world, to prevent their baby developing severe bronchiolitis. See Yellow and Other Virus and Vaccine Perils.
Attempts to make a vaccine for children never fully recovered from Pfizer’s disastrous experiment with formalin-inactivated RSV in 1966. Their “Lot 100” was responsible for several children developing Vaccine-Associated Enhanced Respiratory Disease (VAERD) – from which two died.
There was a three-horse race between Pfizer, GlaxoSmithKline (GSK) and Novavax to devise a vaccine to be given in pregnancy.
Novavax’s RSV vaccine ResVax fell at the first fence – it was ineffective. Pfizer’s MATISSE and GSK’s GRACE (Mat-009) trials were now neck and neck in the maternal phase 3 vaccine championship. Both had similar products – Pfizer’s was RSVpreF and GSK’s RSVpreF3. PreF refers to protein antigens from RSV viruses in the pre-fusion form.
Fall from GRACE
All had been going swimmingly with the GSK trial. Since November 2020 5,300 pregnant people had been enrolled of whom two thirds had received the active vaccine. Until the data monitoring committee spotted worrying data:
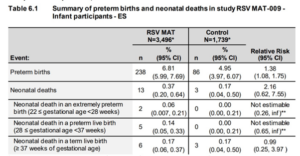
These results showed an obvious imbalance in the number of premature births and neonatal deaths in the vaccinated group. GSK, advised by their independent Data Monitoring Committee acted responsibly. They notified the regulatory bodies (FDA, MHRA, EMA etc.), stopped recruiting new participants and said that they notified all existing participants of this safety (= danger) signal. Where they were irresponsible is that they did not publicly disclose the reason for stopping the trial for twelve months.
Remarkably, there is evidence that investors quickly found out about the premature births and neonatal deaths. Steve Scala, of TD Cowen, an American multinational investment bank, asked about GSK’s analysis of the likely causes of these pre-term births at a conference on 7 March 2022.
Pfizer Clearly Ahead
With a walkover in sight, the Pfizer trial gathered momentum. As with their Covid Trials, there were problems at Ventavia where a couple of Pfizer sites were shut down for irregularities but this would not impede progress – See Boston Strangler.
In April 2022, Phase II results were promoted in the The New England Journal of Medicine (NEJM) which:
“employs a highly rigorous peer-review process to evaluate manuscripts for scientific accuracy, novelty, and importance.”
Rather the Phase II results, this paper was in fact Phase II Interim Analysis. The final data looked somewhat different and are not in the journal.
At the same time that Peter Sellers was keeping us all in stitches, another UK comedian Eric Sykes, usually teamed up with his “sister” Hattie Jacques, who would plaintively cry “Oh Eric” at each demonstration of her brother’s ineptitude.
Oh Eric Phase I
Eric Rubin, Editor-in-Chief of the NEJM, dropped his guard when he allowed Pfizer’s promotion piece to be published. The paper says:
“Fifteen of 403 infants (3.7%) across all the groups were born prematurely, most in the 36th week of gestation”.
The authors and ghosts and peer reviewers presumably omitted to point out that 14 of the 15 were in the vaccinated group, and further analysis showed that the mean incidence of preterm births in all the vaccinated was 4.3% compared with 1.3% in the placebo group.
Oh Eric Phase 2
Although Pfizer employee Kristen Friedman, or someone else, likely ghost-wrote the report, the lead author is pediatrician Prof Eric Simões, owner of Samshoma Medical Research Inc. and also employed by the University of Colorado.
Eric S had a foot in both camps, for at the same time that he was a Principal Investigator for Pfizer’s RSVpreF vaccine trial, he was a paid member of GSK’s Data Monitoring Committee for their RSVpreF3 trial!
He kept mum. The Phase II article contains no reference to the preterm births and neonatal deaths problem in GSK’s trial, a problem that Eric, you’d assume, knew all about.
Eric S also co-authored the latest Phase 3 Pfizer report (coincidentally also published in the NEJM) and again GSKgate is not referred to. Eric S probably wasn’t aware of Pfizer’s raw data; data that showed twice as many infants with “severe or life-threatening” neonatal jaundice in the vaccinated group, twice as many premature births before 34 weeks’ gestation, and showed that the vaccinated had a statistically significant excess of “severe or life-threatening” pre-eclampsia within one month of their shot.
Eric S’s papers in Eric R’s NEJM are curiously silent on breastfeeding. In 2011 Eric S wrote:
“The role of breast-feeding in preventing RSV disease and hospitalization for RSV is undisputed.”
He wrote this article in conjunction with Constanze Sommer, possibly a distant cousin of Peter Sellers’s glamorous co-star Elke Sommer.
Read all about it
There has been a recent epidemic of news items where journalists have shared portents of doom: the end of the world is nigh.
Of course readers of this blog will recall that the first RSV vaccines were created over 50 years ago.
Not a plug for Pfizer

The image above is taken from the website of the London School of Hygiene and Tropical Medicine (LSHTM) – a UK charity. Beate was the lead author of Pfizer’s Phase 3 trial to protect babies from the “potentially deadly” virus. She claims that the results were “absolutely excellent”.
Deadly, How deadly?
Not in the higher income countries where Pfizer hope the vaccine will be first approved. In 2016 the Gates foundation stumped up $M2.5 to establish a global mortality registry for respiratory syncytial virus to track vital information – RSV Gold – which seems to have given up on their task.
A recent journal article points out that in Finland, the number of RSV-related deaths extracted for the years 2000–2018 was too small to be shared due to the European Union’s GDPR. And in Scotland, with one of the higher mortality rates, 10 RSV-related deaths were identified in children under two years of age between 2010–2016. Most deaths are in children with severe underlying other conditions. And the deaths occur at most at a rate of 69/100,000 hospitalizations.
Data manipulation.
Pfizer’s two presentations, at ACIP and RSVVW, were virtually identical. Beate proclaimed the Good News in Lisbon while Pfizer’s Iona Munjal spread the Gospel at ACIP.
But one of Pfizer’s slides was missing at the Lisbon meeting.
How do you best display scary data about early preterm births? The starting point is the incidence: 20/3568 vs 11/3558. Put another way, an 81.8 percent increase in the vaccinated group. Greater than the success rate of preventing RSV disease in the babies. You must disguise it graphically.
This is where Inspector Clouseau’s magnifying glass comes in handy.

Can’t spot the problem? That’s the whole point. It’s the column on the very left that gives you the impression there is no problem. But this is generated by 2 tricks. One is the Y-Axis trick which shrinks smaller numbers down so they barely show against a huge axis. The other is converting the numbers to percentages.
We have converted the Y axis to 250 units rather than 100 units, which should hide Pfizer’s problem even better but also converted the percentages back to numbers and now on the Left you see the problem they don’t want you to notice.
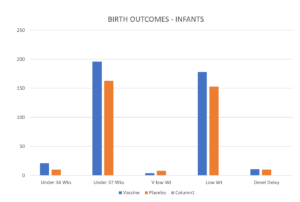
Beate’s Lisbon presentation didn’t show this slide. But she was asked about prematurity by the chair, a BFG employee, philatelist and bon viveur Keith Klugman. Looking down at her notes, she dashed off a quick answer about there being no statistically significant difference – and there was less prematurity overall than would be expected so participating in trials is good for people.
She didn’t mention that to participate you couldn’t have had a prior history of prematurity or any other problems in pregnancy.
The Klugman – Beate exchange looks stage-managed.
Before asked the question, Klugman said he was so thrilled with the results and excitedly announced that the Gates Foundation had given Pfizer a large donation ($27.5 million) to enable multidose vials of the RSVpreF vaccine to be manufactured for use in lower income countries.
- This is a vaccine that does not yet have regulatory approval.
- It has an adverse event profile that merits close examination.
- It is not clear if it works in a meaningful sense.
- There is no case for encouraging Western women to have it
- Should Westerners decide whether it is used widely in Low or Middle Income Settings
The world of RSV prevention is dominated by doctors, who regularly get themselves tied up in knots trying not to say “pregnant women”. Will this language work in Low and Middle Income settings?



Shots in the Light – ‘A beump’ …
RSV Gold
“A potential new weapon…..https://www.youtube.com/watch?v=ebkY0u1-NKk “my hands are lethal weapons……
Pfizer RSV vaccine safe for pregnant women, company says
https://www.youtube.com/watch?v=PYEM4xa9Y-I
ADVISORY COMMITTEE MEETING
Vaccines and Related Biological Products Advisory Committee May 18, 2023 Meeting Announcement
https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-may-18-2023-meeting-announcement
‘Agenda’ – pregnant individuals
The meeting presentations will be heard, viewed, captioned, and recorded through an online teleconferencing platform. On May 18, 2023, the committee will meet in open session to discuss and make recommendations on the safety and effectiveness of ABRYSVO (Respiratory Syncytial Virus Vaccine), manufactured by Pfizer Inc., with a requested indication, in Biologics License Application (BLA) 125768 (STN 125768/0), for the prevention of lower respiratory tract disease and severe lower respiratory tract disease caused by RSV in infants from birth through 6 months of age by active immunization of pregnant individuals.
No waivers were issued for conflicts of interest for this meeting.
Global South – ‘A beump’ …
https://qz.com/rsv-vaccine-pfizer-global-south-1850401425
“It’s an issue of priorities and incentives within the company about what matters—and what matters is prioritizing profit again,” she says.
‘A beump’ …
‘Worthy of urgent attention’ Women will be giving birth without knowing what the risks of vaccinations are or of the likelihood of being prescribed ADs if and when they do find out.
09 May 2023
Robin J Forrest
PhD Student
Huseyin Naci
The London School of Economics
Houghton St, London
Respond to this article
Read all responses to this article
Antidepressants and suicidality: scrutiny required
Re: Suicide in young people: screening, risk assessment, and intervention John V Campo, Jeffrey A Bridge, et al. 381:doi 10.1136/bmj-2022-070630
Dear Editor
It is concerning that Hughes and colleagues’ review of suicide in young people (1) neglects the importance and complexity of the relationship between suicidality and antidepressants. That some depressed patients become more rather than less suicidal with pharmacotherapy has been recognized since the launch of the tricyclic imipramine in the 1950s. This seemingly paradoxical effect became more obvious with serotonin reuptake inhibitors in the late 1980s, notably in the young (2) and led to ‘black box’ warnings. Claims that resulting prescribing restrictions have prevented needed treatment, causing youth suicide rates to increase, have been refuted by careful time-course analyses (3).
In particular, we are concerned by the reliability of Hughes and colleagues’ analysis. For example, their senior author concluded in 2007 (their ref. 138) “there was increased risk difference of suicidal ideation/suicide attempt across all trials and indications for drug vs placebo” but this was downplayed in the present review which notes only a “small risk difference”. Hughes and colleagues also cite a paper reviewing industry sponsored trials (their ref. 139), purporting to find “no increases in treatment-emergent suicidality” without addressing the significant shortcomings in methodology and reporting of pivotal trials (4). Finally, Hughes and colleagues do not cite or comment on a recent BMJ systematic review concluding that “in children and adolescents the risk of suicidality and aggression doubled” in response to antidepressants compared to placebo (5).
Efforts to prevent, monitor and manage antidepressant-induced suicidality (6) have not yet provided evidence for safe clinical practice, despite Hughes and colleagues’ claims (1). Considering the high prevalence of antidepressant prescription and an estimated Number Needed to Harm for suicide of approximately 1300 (7), this is a public health issue worthy of urgent attention.
References
1. Hughes JL, Horowitz LM, Ackerman JP, Adrian MC, Campo JV, Bridge JA. Suicide in young people: screening, risk assessment, and intervention. BMJ. 2023;381:e070630. doi:10.1136/bmj-2022-070630
2. Hetrick SE, McKenzie JE, Cox GR, Simmons MB, Merry SN. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2012;11(11):CD004851. doi:10.1002/14651858.CD004851.pub3
3. Stone MB. The FDA warning on antidepressants and suicidality–why the controversy?. N Engl J Med. 2014;371(18):1668-1671. doi:10.1056/NEJMp1411138
4. Gøtzsche PC, Healy D. Restoring the two pivotal fluoxetine trials in children and adolescents with depression. Int J Risk Saf Med. 2022;33(4):385-408. doi:10.3233/JRS-210034
5. Sharma T, Guski LS, Freund N, Gøtzsche PC. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352:i65. doi:10.1136/bmj.i65
6. Healy D, Bechthold K, Tolias P. Antidepressant-induced suicidality: how translational epidemiology incorporating pharmacogenetics into controlled trials can improve clinical care. Per Med. 2014;11(1):79-88. doi:10.2217/pme.13.93
7. Hengartner MP, Plöderl M. Newer-Generation Antidepressants and Suicide Risk in Randomized Controlled Trials: A Re-Analysis of the FDA Database. Psychother Psychosom. 2019;88(4):247-248. doi:10.1159/000501215
Competing interests: No competing interests
09 May 2023
Alain Braillon
retired senior consultant
Brian D. Earp (Senior Research Fellow, Uehiro Centre for Practical Ethics, Faculty of Philosophy, University of Oxford, Oxford, England), David Benjamin Menkes (Academic psychiatrist, Department of Psychological Medicine, University of Auckland, New Zealand)
80000
A Shot in the Moonshots…
Albert Bourla
@AlbertBourla
Scientists have tried to develop RSV vaccines for more than 50 years without success. But now we’re on the cusp of a scientific renaissance where the seemingly impossible is now potentially possible. Case in point: Great progress on our 2 RSV investigational vaccine trials.
Pfizer Inc.
@pfizer_news
Results from two Phase 3 studies of our respiratory syncytial virus (#RSV) vaccine candidate, RSVpreF, have published in @NEJM 1/3
https://twitter.com/pfizer_news/status/1643724570011025408
MATISSE/RENOIR…
Brook Jackson
@IamBrookJackson
Be sure you keep an eye on GSK @elonmusk ! I would hate to see your second-generation mRNA vaccine candidate explode like that next-generation Starship of yours did several weeks back.
4/20 blows sometimes, huh.
Brook Jackson
@IamBrookJackson
·
CureVac, in collab w/GSK, announced this am that they have dosed the first patient in a Phase 1/2 study of an mRNA flu vaccine. Strategic partnerships include the Bill & Melinda Gates Foundation, CEPI, CRISPR, Genmab & Harvard Medical School. @elonmusk
https://www.curevac.com/en/about-us/partnerships/
Brook Jackson
@IamBrookJackson
Dear @Pfizer and @GSK
—I own you both now! From COVID to RSV, some of us are not afraid to stand up to tell the truth! The misconduct, fraud and every violation of federal law will be exposed.
You are a threat to public safety!
A Shot In the Dark:
https://davidhealy.org/a-shot-in-the-dark-for-pregnant-people/
A Shot in the Dark…
“on the cusp of a scientific renaissance” …
“Many suicidal events on paroxetine had been omitted or given an obscure name such as emotional lability. I consider this fraud,” he added.
Prozac “unsafe & ineffective” for young people, analysis finds
Reanalysis of regulatory data finds that attempted suicides were excluded from the final journal publication, and the journal has not corrected the record.
MARYANNE DEMASI, PHD
10 MAY 2023
https://maryannedemasi.substack.com/p/prozac-unsafe-and-ineffective-for?utm_source=post-email-title&publication_id=1044435&post_id=119202332&isFreemail=true&utm_medium=email
A new analysis finds that Prozac (generic name fluoxetine) is unsafe and ineffective for treating depression in children and adolescents.
Regulatory documents show that trial participants attempted suicide after taking fluoxetine, but these events were excluded from the final journal publication.
I notified the journal of the new findings, but the editor refuses to correct the record.
Prozac approval
In 2002, Prozac (fluoxetine), manufactured by Eli Lilly, was FDA-approved for the treatment of depression in children and adolescents based on data from two clinical trials.
The two trials were published in peer-reviewed journals in 1997 (Study 1) and 2002 (Study 2).
Both publications reported a small benefit of fluoxetine over placebo in young people with depression and there appeared to be no major safety concerns.
Subsequently, fluoxetine became one of the most prescribed antidepressants for children aged 0-19 years in the US, and is in the top 5 most prescribed antidepressants in England.
Restoring old trials
An initiative called Restoring Invisible and Abandoned Trials (RIAT) has enabled researchers to “restore” old clinical trial publications by analysing documents submitted to drug regulators by the drug companies.
These analyses have revealed that serious drug harms are either underreported or excluded entirely from medical journals.
Physician Peter Gøtzsche and psychiatrist David Healy obtained regulatory documents (protocols and clinical study reports) from UK’s drug regulator (MHRA) of the two fluoxetine trials that underpinned the drug’s approval in 2002.
The discrepancies
Multiple problems were identified when Gøtzsche and Healy compared the clinical study reports of the two fluoxetine trials, with what was published in the medical journals.
Many suicidal events in people taking fluoxetine were either missing or labelled incorrectly in the published reports.
For example, in Study 1, the clinical study report described two patients who’d attempted suicide after 12 and 15 days of taking fluoxetine, but these events were excluded from the journal article.
They found problems with ‘blinding’ in both trials, meaning the trial investigators were likely aware of which patients were on the drug or the placebo.
They also found that people who were recruited into the trial, and who were already taking an antidepressant, were only given one week to “wash out” the drug from their system before commencing the randomisation process.
This caused severe withdrawal symptoms in some participants who ended up in the placebo group, making it difficult to ascertain the true level of harms in the treatment group.
Finally, when Gøtzsche and Healy looked back and analysed the data from the primary outcome – which was depression – there was no meaningful benefit from fluoxetine compared to placebo.
Journals turn a blind eye?
I wrote to both journals asking if the editors would consider correcting the discrepancies and clearly delineate the adverse events that were not reported in the published articles through an erratum.
Neither journal has done so.
The editor at Arch Gen Psychiatry (now called JAMA Psychiatry) rejected concerns about two suicide attempts that were omitted from its publication of Study 1, and has not made any corrections or clarifications.
In response, Gøtzsche said, “It’s totally unacceptable. When attempted suicides are left out of journal articles, which has happened in many such trials, it changes the safety profile of the drugs completely. This is important information that patients should know about before considering taking the pills.”
Gøtzsche drew similarities to another placebo-controlled trial in adolescents which used the drug Paxil (paroxetine).
GlaxoSmithKline’s Study 329 famously claimed that “Paroxetine is generally well tolerated and effective,” but when researchers restored the trial data using regulatory documents, the opposite became true.
“A restoration of the data from Study 329 showed that paroxetine was neither safe nor effective for treating depression in children and adolescents,” said Gøtzsche.
“Many suicidal events on paroxetine had been omitted or given an obscure name such as emotional lability. I consider this fraud,” he added.
The editor at J Am Acad Child Adolesc Psychiatry (JAACAP), which published Study 2 of fluoxetine said they would not respond to criticisms until the discrepancies documented by Gøtzsche and Healy were published in a peer-reviewed journal.
The process took over a year, but Gøtzsche and Healy’s paper has now been published in a peer-reviewed journal and sent to the JAACAP for review.
The JAACAP said in a statement:
JAACAP takes seriously its responsibility to ensure scientific integrity. As stated in the guide for authors, review of post-publication critiques will be managed according to Committee on Publication Ethics (COPE) guidelines. We will let you know the outcome of the review process…
Why does it matter?
The restoration of old trials has revealed to patients and physicians that much of the data in peer-reviewed journals are incomplete, biased, and often cherry-picked.
The exclusion of suicide attempts and suicides distorts the medical literature and prescribing guidelines to such an extent that they cannot be trusted. It also may reduce options for safer, more effective interventions such as psychotherapy.
“I’ve heard from many families whose children committed suicide because of antidepressants. We should not be prescribing them to young people,” said Gøtzsche.
“Our meta-analysis of ten trials showed that psychotherapy halved the occurrence of new suicide attempts in patients admitted after a suicide attempt. Psychotherapy is what they should be getting, not pills,” he added.
Ultimately, it’s the patients who pay the price, sometimes with their lives, from distorted clinical data, and from journals that refuse to correct glaring errors.
Antidepressants like fluoxetine double the risk of suicide and aggression in children and adolescents, they often lead to decreased quality of life, they cause sexual dysfunction in about 50% of users, and these harms may continue long after they try to quit.
In conclusion, there seems to be no rationale for using fluoxetine in young people for treating depression – the new analysis concludes the drug is unsafe and ineffective.
“This is where Inspector Clouseau’s magnifying glass comes in handy.” …
A Shot in the Can…
“Coke and Pepsi”
Brook Jackson
@IamBrookJackson
·
6h
BREAKING: Anyone remember that BMJ investigation from 2021 “Covid-19: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial” How about a new analysis on Maternal RSV vaccines?
https://bmj.com/content/381/bmj.p1021…
https://twitter.com/IamBrookJackson/status/1656445471643009024
Maternal RSV vaccine: Further analysis is urged on preterm births
BMJ 2023; 381 doi: https://doi.org/10.1136/bmj.p1021 (Published 10 May 2023)Cite this as: BMJ 2023;381:p1021
https://www.bmj.com/content/381/bmj.p1021
After the safety signal in the GSK study came to light, experts have questioned Pfizer over the possibility of an increased risk of preterm birth in its trials. On investor calls in October 20229 and March 202310 Steve Scala, a pharmaceutical industry research analyst and former pharmacist, described Pfizer’s and GSK’s vaccines as “Coke and Pepsi” and said he was “wondering why Pfizer can continue and feel comfortable” given that there didn’t seem to be a reason for a different incidence of preterm births in the two studies.10 On both occasions, Pfizer representatives did not give reasons why their vaccine would perform differently to GSK’s.
Brook Jackson
@IamBrookJackson
·
59m
Ventavia’s personalized messaging to potential maternal RSV clinical trial participants. How did they get these patient records? Paid OBGYN friends $1,500 bucks for every patient enrolled violating the Anti-Kickback Statute.
https://twitter.com/IamBrookJackson/status/1656526984867094541
I’M REALLY PISSED OFF and believe it or not, there’s more to come.
https://twitter.com/IamBrookJackson/status/1656190429224910848?s=20
PUBLIC SUBMISSION
Comment from Peter Selley
Posted by the Food and Drug Administration on May 9, 2023
https://www.regulations.gov/comment/FDA-2023-N-0378-0010
FDA-2023-N-0378 Pfizer’s ABRYSVO (RSVPreF) vaccine in pregnancy
I refer to the NEJM reports of the Phase 2 and Phase 3 studies.
It is remarkable that the FDA:
1) – 7)
Please read the single attached document detailing my concerns about safety considerations for this vaccine in pregnancy.
I have shared it with representatives of Pfizer in preparation for this meeting.
All current FDA comments, Pfizzing on the site…
A Shot in the Mothers-To-Be…
Pfizer’s RSV vaccine given to mums-to-be may raise the risk of premature birth, experts warn
No safety concerns were alerted by Pfizer in their own phase three trial
But experts suggested the data should be reassessed and analysed further
In February GSK halted its trial after finding a raised risk of premature births
https://www.dailymail.co.uk/health/article-12068769/Pfizers-RSV-vaccine-given-mums-raise-risk-premature-birth-experts-warn.html
Fears were raised today that a Pfizer vaccine given to mothers-to-be could raise the risk of babies being born prematurely.
Experts have called for tougher scrutiny over the new respiratory syncytial virus (RSV) jab, which could be approved in the US and UK later this year.
It comes as GlaxoSmithKline halted its own phase three trial of its maternal RSV vaccine in February after discovering an increased risk in premature births in vaccinated mothers.
No safety concerns were alerted by Pfizer in its own phase three trial, considered the final hurdle before approval by health bodies.
Yet experts today told The BMJ, which first reported the concerns, it was important to reassess and analyse the findings using more sensitive measures.
RSV is common respiratory virus that usually causes mild, cold-like symptoms, normally in winter.
It is part of the same family as the mumps and measles viruses.
Pfizer’s maternal RSV vaccine aims to protect infants from severe illness caused by the virus. Though it is not yet approved, it would be offered to women who are six to nine months pregnant, if approved.
RSV is very common but can be fatal, especially in young children.
In 2019, an estimated 3.6 per cent of all deaths worldwide in children aged one to six months were due to RSV, with 97 per cent of these deaths occurring in low and middle income countries.
The vaccine has not yet been approved for use, but a decision by the US Food and Drug Administration (FDA) is expected by August.
The European Medicines Agency is also set to make an approval decision later this year.
In results of an interim analysis of its phase three trial last month, Pfizer said the vaccine was effective against medically-attended severe RSV in children.
No statistically significant differences were identified in premature births, it confirmed.
However, experts have suggested the data should be analysed further while the FDA should also carry out post-approval monitoring, if approved.
Professor Klaus Überla, director of the Virological Institute of the University Hospital Erlangen and member of the RSV working group of the Standing Committee on Vaccination, told the BMJ: ‘My interpretation of all these data is that there may be a safety signal for preterm births that should be followed up on.’
Meanwhile, Professor Cody Meissner, a paediatrician at the Dartmouth Geisel School of Medicine and consultant in the US Centers for Disease Control and Prevention (CDC)’s maternal RSV working group, predicted possible adverse effects such as premature births will be ‘closely monitored’ in assessment programs by FDA and CDC.
He added: ‘We need a safe vaccine.’
Another scientist at the US National Institutes of Health, who was not authorised to speak with the media, also recommended Pfizer data should be analysed using more sensitive measures, such as average birth weight and subgroup analyses to detect possible signals.
Work on an RSV vaccine has met roadblocks for decades following multiple failures in clinical trials.
This includes one study in the 1960s, where children who received an experimental vaccine experienced worse disease than those in the placebo arm. Two youngsters, both under the age of two years, died.
Research later found that this was because the vaccine had targeted a version of the protein on the virus that forms after it fuses to a cell, meaning it did not trigger protection against the disease and may have actually hampered the immune response.
In February, GSK halted its phase three trial into its maternal RSV jab, citing safety concerns.
In a document submitted to the FDA, GSK’s data showed an increase in premature births in vaccinated mothers (6.8 per cent) when compared to those who received the placebo (4.9 per cent).
It accounted for one extra premature birth in every 54 vaccinated mothers.
Some 13 neonatal deaths were also recorded among those who were vaccinated.
Just three were reported in the placebo group.
Ilse Dieussaert, vice president of vaccine development at GSK, however said the increase in neonatal deaths was due to deaths in premature babies.
There was no imbalance of deaths in full term babies, she noted.
The number of babies born prematurely was greatest in low and middle income countries, she noted. Almost no difference was observed in high income countries.
Earlier this week the first-ever vaccine against RSV was approved in the US by the FDA, after it gave the green light for the vaccine to be administered to adults aged 60 years and older.
Brook Jackson
@IamBrookJackson
·
2h
In Pfizer’s maternal COVID vaccine study, there were 68 US sites, represented in 13 different states & Ventavia just so happens to be featured on this national morning news show.
@CBSMornings
https://twitter.com/IamBrookJackson/status/1656564209939886081
Show Thread
“It just seemed like a win-win”
Science Will Win
@Pfizer…
Calls to monitor Pfizer’s results
Maternal RSV vaccine: Further analysis is urged on preterm births
BMJ 2023; 381 doi: https://doi.org/10.1136/bmj.p1021 (Published 10 May 2023)
Cite this as: BMJ 2023;381:p1021
Linked Editorial
Preventing respiratory syncytial virus bronchiolitis in infants
Responses
Hristio Boytchev
Author affiliations
A “safety signal” in a similar respiratory syncytial virus (RSV) vaccine has led to trials being stopped and prompted calls for a cautious approach to using the vaccine in pregnant women, reports Hristio Boytchev
Experts have called for further analysis of trial data and post-approval monitoring of Pfizer’s maternal RSV vaccine candidate after GSK’s trials of a similar product were halted over a rise in preterm births and neonatal deaths.
An advisory committee from the US Food and Drug Administration (FDA) is set to discuss the vaccine on 18 May1 as part of a fast-tracked priority review, with a decision expected by August.
Pfizer published the results of an interim analysis of its phase 3 trial2 in April 2023, saying that the vaccine was effective against medically attended severe RSV in children and that no safety concerns were identified.
But the results have raised concerns34 about a possible increase in preterm births, and experts are calling for further analyses of the data and for post-approval monitoring of the vaccine, should the FDA approve it.
GSK halts its trials
In February 2022, GSK halted enrolment and vaccination across three phase 3 trials of its maternal RSV vaccine candidate, citing a safety signal in one of them.5 It emerged that the concern was about an increased risk of preterm birth in the vaccine arm.6
In a document submitted to the FDA, GSK’s data showed 238 preterm births out of 3496 (6.8%) in the vaccine arm and 86 out of 1739 (4.9%) in the placebo arm—around one extra preterm birth for every 54 vaccinated mothers.7 There were 13 neonatal deaths in the vaccine arm and three in the placebo arm.7
GSK said it is still investigating the cause of the preterm births and presented preliminary findings in a conference presentation earlier this year.8
Ilse Dieussaert, vice president of vaccine development at GSK, said that the increase in neonatal deaths was because of deaths in premature babies and that there was no imbalance of deaths in full term babies. Dieussaert explained that the preterm imbalance was greatest in low and middle income countries (LMIC), which had 9.9% preterm births in the vaccine and 6.3% in the placebo arm. Almost no difference was observed in high income countries.
According to GSK’s analysis, the difference in preterm births was highest in LMIC in women who had decided to have different additional vaccines, with 8.2% in the vaccine arm compared with 4.3% in the placebo arm. None other of the factors analysed could explain the safety signal, including SARS-CoV-2 infections, Dieussaert said.
“Similar” vaccine
Pfizer’s vaccine is similar to GSK’s, although there may be differences in manufacturing, says Cody Meissner, professor of paediatrics and medicine at the Dartmouth Geisel School of Medicine and consultant in the US Centers for Disease Control and Prevention’s (CDC) maternal RSV working group. Both are subunit vaccines using a recombinant RSV F protein of the virus, stabilised in its prefusion state. “I can’t really give you an idea as to why one would cause a problem and the other one wouldn’t,” Meissner said.
After the safety signal in the GSK study came to light, experts have questioned Pfizer over the possibility of an increased risk of preterm birth in its trials. On investor calls in October 20229 and March 202310 Steve Scala, a pharmaceutical industry research analyst and former pharmacist, described Pfizer’s and GSK’s vaccines as “Coke and Pepsi” and said he was “wondering why Pfizer can continue and feel comfortable” given that there didn’t seem to be a reason for a different incidence of preterm births in the two studies.10 On both occasions, Pfizer representatives did not give reasons why their vaccine would perform differently to GSK’s.
Differences in preterm births are evident in Pfizer’s RSV trial. In adverse event tables for its phase 2 study, published in October 2022, Pfizer reported 3 out of 116 (2.6%) premature births in the placebo group and 6 out of 114 (5.3%) in the group that received the vaccine that was chosen as Pfizer’s final product.11
In a prespecified interim analysis of Pfizer’s related phase 3 trial published in April, 201 babies (5.6%) were born prematurely to vaccinated mothers v 169 babies (4.7%) in the placebo group.12 According to the protocol of the trial, Pfizer was studying preterm birth as an “adverse event of special interest.”12
Pfizer did not respond when asked about a possible increase in preterm births associated with the vaccine in its two trials, but told The BMJ that “no imbalance of neonatal deaths was observed” in its phase 3 trial.
Calls to monitor Pfizer’s results
While the difference in preterm births in the Pfizer trials was not statistically significant, it should be reviewed in light of the signal seen in GSK’s trial, experts told The BMJ.
“My interpretation of all these data is that there may be a safety signal for preterm births that should be followed up,” said Klaus Überla, director of the Virological Institute of the University Hospital Erlangen and member of the RSV working group of the Standing Committee on Vaccination (STIKO), which develops national recommendations for the use of licensed vaccines in Germany.
After a possible approval, the vaccine manufacturers will probably be obliged to monitor a much higher number of pregnancies than were studied in the trials, said Fred Zepp, vice president of the Association of the Scientific Medical Societies in Germany and member of the STIKO RSV working group. (Zepp stressed he was speaking as a researcher and not on behalf of STIKO).
The Pfizer data should be analysed using more sensitive measures such as average birth weight and subgroup analyses to detect possible signals, said a scientist at the US National Institutes of Health who was not authorised to speak with the media.
Meissner predicts that possible adverse effects such as premature births will be “closely monitored” in assessment programmes by FDA and CDC. “We need a safe vaccine,” he added.
Footnotes
This news story has been funded by the BMJ Investigations Unit. For details see bmj.com/investigations.
A Shot in the FDA…
Comment Period ends in 7 days
Vaccines and Related Biological Products Advisory Committee; Notice of Meeting; Establishment of a Public Docket; Request for Comments
Posted by the Food and Drug Administration on Apr 11, 2023
https://www.regulations.gov/document/FDA-2023-N-0378-0004/comment
Comment from Craig Evans
I am a retired family practice physician and I am NOT in favor of an RSV vaccine given to pregnant women to prevent the disease in newborns. I am very hesitant for new vaccines given to pregnant women given the terrible reporting of complications of the CoVid vaccine given to pregnant women with increase of spontaneous abortions and reduced fertility. The scientific community has lost much of its trust and, I think, justifiably so over the failure to adequately inform the public with ALL the evidence and expect adults to make the right decisions for themselves. We need to reestablish a reliable and consistent advisory role in our practice of preventive and active treatment regimens.
Comment from Judith Caldwell
Docket No. FDA–2023–N–0378 for “Vaccines and Related Biological Products Advisory Committee (VRBPAC); Notice of Meeting; Establishment of a Public Docket; Request for Comments.
Please consider ALL facts and evidence concerning RSV and the Pfizer drugs produced to treat RSV. Please do not limit yourselves to the materials provided for consideration by Pfizer.
I have attached for your review other additional relevant studies and facts that you may not otherwise be provided with to assist in making your decision on May 18, 2023.
Thank you!
Download: Extract:
Reactions, Deaths of Pregnant Women and Babies in RSV Vaccine Clinical Trials
The ACIP has also reviewed data presented by Pfizer on ABRYSVO for use in pregnant women as a single dose given at 24‒36 weeks during pregnancy. The rationale offered by public health officials is that injecting pregnant women with RSV vaccine will enable them to pass on protective antibodies to their unborn children to prevent RSV in infants from birth through six months of age.16
Data presented by Pfizer from RSV clinical trials involving 3,682 vaccinated pregnant women revealed that nearly 14 percent experienced a vaccine adverse event, with 4.2 percent reported as serious, 1.7 percent reported as severe, and 0.5 percent as life-threatening.17
Pfizer also provided data that 37.1 percent of infants, whose mothers received the experimental RSV vaccine during pregnancy, were reported to have experienced an adverse event within one month of birth. Of these reports, 15.5 percent were reported as serious, 4.5 percent as severe, and 1 percent as life-threatening.18 Pfizer data also noted small increases in premature and low birth weight for infants born to RSV vaccinated pregnant women. The RSV clinical trial data also included the death of one pregnant woman, 18 still births (10 in vaccinated pregnant women and 8 in unvaccinated pregnant women), and 17 infant deaths (5 from the vaccinated pregnancy group and 12 in unvaccinated pregnancy group).
Pfizer stated that all deaths occurring in the RSV vaccine clinical trials were unrelated to the vaccine.19
Once approved for use in the general population for pregnant women, associated RSV vaccine injuries to the mother and/or unborn child are subject to filing injury claims with the federal VICP,20 which essentially provides vaccine makers with a total liability shield when their vaccines cause injury and death. It is unclear at this point in time whether the RSV vaccine will be recommended for administration during each pregnancy.
More ‘Coke’ in the ‘Can’…
A Shot in the Mirth…
The Rise and the Rise of Pfizer, who, in fact, have to do very little
Just send it to Eric…
Since the pandemic, Brits are dying in their thousands and no one knows why
“Britain’s getting older, and gaining a larger average body-mass index.”
He said: “The population is getting older, and also the population in Britain is the fattest in Europe
https://www.msn.com/en-gb/news/other/since-the-pandemic-brits-are-dying-in-their-thousands-and-no-one-knows-why/ar-AA1b4OH9?ocid=msedgdhp&pc=U531&cvid=55492c2596f2495b8fd3b0e51879ba61&ei=9
Dr Aseem Malhotra Retweeted
James Thorp MD
@jathorpmfm
·
8h
Leading OB-GYN Group Took $11 Million From CDC to Push COVID Shots on Pregnant Women, Documents Reveal • Children’s Health Defense
The Centers for Disease Control and Prevention bankrolled the American College of Obstetricians and Gynecologists to the tune of $11 million to promote COVID-19 vaccination as “safe and effective” for pregnant women, according to an investigation published this week by attorney Maggie Thorp.
https://childrenshealthdefense.org/defender/obgyn-cdc-covid-vaccine-pregnant/
“The Department of Defense, HHS, CDC, ACOG, the Society for Maternal-Fetal Medicine, the American Board of Obstetrics and Gynecology and others are unequivocally attempting to abolish a highly-revered, God-ordained, gold standard doctrine that has been memorialized and honored and has withstood the scientific test of time over millennia: Never give novel substances in pregnancy without short-term and long-term outcome studies in the offspring.
“I will not allow this charade to continue. The reproductive toxicology studies are damning as evidenced by Alexandra Latypova, a 30-year veteran of the pharmaceutical industry demonstrating major problems — miscarriages, birth defects and many other concerns.
“Did not these governmental agencies and medical organizations learn their lessons from the thalidomide and diethylstilbestrol [DES] disasters of the last century? The COVID-19 gene therapies make thalidomide and DES look like prenatal vitamins.
“If one does not understand history they will be destined to repeat it on an even grander scale.”
Brook Jackson Retweeted
Robert F. Kennedy Jr
@RobertKennedyJr
·
In my house, we watch the Television news not to learn the truth but to understand the official narrative.
“A paper published in the prestigious New England Journal of Medicine”
RSV vaccine for pregnant women provides protection for babies: study
https://www.uwa.edu.au/news/Article/2023/April/RSV-vaccine-for-pregnant-women-provides-protection-for-babies-study
“In the next 10 years I hope to see licensed vaccines and preventative drugs being given to mothers, babies, toddlers and older adults, with multiple vaccine and monoclonal antibody platforms available that could even be combined with COVID and influenza vaccines, helping keep our hospitals and GP surgeries much quieter over winter.”
https://twitter.com/kosta_mira/status/1656710396177162240
“With a walkover in sight, the Pfizer trial gathered mom entum…
A Shot in the Astra-Zeneca…
Daily Mail Full-Coverage
“No-one has been paid out for injuries or deaths relating to Pfizer or Moderna’s jab so far.”
The £1billion battle for Covid jab justice: How 90 British families left bereaved or disabled after getting AstraZeneca’s vaccine are fighting for compensation to avoid financial ruin… and they insist they are NOT anti-vax
The vaccine injured could need ‘multi-million pound’ payouts due to disability
https://www.dailymail.co.uk/health/article-12059245/Inside-1billion-AstraZeneca-compensation-battle.html
UPDATED: 16:03, 13 May 2023
Britain’s compensation bill for victims killed or maimed by AstraZeneca’s Covid jab could theoretically exceed £1billion, MailOnline can reveal.
Around 90 families are currently pursuing legal action against the pharmaceutical titan, claiming the jab was essentially a defective product.
Lawyers representing the claimants believe that some of the cases could be worth up to £20m in compensation, which is roughly 160 times more than the £120,000 Government support available. They hope to be able to prove that the vaccine was to blame in court.
How safe is AstraZeneca’s Covid jab? What are the side effects? How many Brits have died from complications? Our forensic Q&A answers everything you need to know
https://www.dailymail.co.uk/health/article-12015353/Our-forensic-Q-AstraZeneca-jab.html
An 18-year-old aspiring paramedic, a rock musician and an award-winning BBC radio presenter: The ‘victims’ of AstraZeneca’s Covid vaccine
Jab complications were rare but the massive rollout saw dozens of Brits killed
https://www.dailymail.co.uk/health/article-12016301/The-victims-AstraZenecas-vaccine.html
I’m not an anti-vaxxer. I just lost my husband to AstraZeneca’s Covid jab two years ago and have had to sell my home to help pay the bills – because I STILL haven’t got any compensation
Kam Miller, 57, from Leicester, lost her husband Neil to AstraZeneca’s vaccine
Two years since his death she has still received no compensation or support
https://www.dailymail.co.uk/health/article-12015521/Im-not-anti-vaxxer-just-lost-husband-AstraZenecas-Covid-jab.html
Exposed: ‘Cruel’ flaws of Government-funded financial support scheme for Brits injured by AstraZeneca’s Covid vaccine (and don’t even bother trying to claim the one-off payment of £120k if you’re only ‘59% disabled’)
Covid jab deaths and injuries have shown the 1970s scheme isn’t fit for purpose
https://www.dailymail.co.uk/health/article-12016247/Damaged-AstraZenecas-Covid-jab-Universal-Credit-Sorry-youre-not-getting-120-000.html
The Reclaim Party
@thereclaimparty
@thereclaimparty MP @ABridgen has launched his legal Claim against @MattHancock
A Shot in the Dark…
“And the jab itself, while marred by some fatalities, was on the whole a success. Experts estimate it saved 6million lives across the world. Millions more were protected from falling seriously ill.”
“Britain’s Daily Mail, possible the best newspaper for coverage of health issues, is also now covering RSV Vaccines. It will be a long time before the Guardian ever gets round to doing so.”
A Shot…
“For these reasons, NVIC opposes the use of Abrysvo in pregnant women and strongly encourages the VRBPAC and FDA to reject the use of Abrysvo in pregnant women.”
Comment from National Vaccine Information Center
Posted by the Food and Drug Administration on May 12, 2023
https://www.regulations.gov/comment/FDA-2023-N-0378-0011
Attached is the written comment on behalf of the National Vaccine Information Center.
Download: Extract:
Clinical Trial Data and Cost Analysis Do Not Support Approval
Given the above data, Abrysvo RSV vaccine represents an unnecessary risk to mother and child for very meager reductions in RSV cases, severe and typical, as well as hospitalizations, for an illness that represents little threat to healthy infants.
Additionally, the clinical trial sample size appears to be underpowered and is not likely to capture severe vaccine adverse events, such as GBS. Data also suggest that even with the small sample size the vaccine may contribute to undesirable events such as premature birth and low birth weight in infants. While the global market for RSV vaccine has been estimated to be over $10 billion by 2030,27 it makes little fiscal sense for the government to expend monies for use of Abrysvo vaccine in pregnant women under the guise of providing protection for infants, particularly when there is no correlate of protection for RSV and trial data demonstrates the vaccine will not prevent the transmission of the illness. For these reasons, NVIC opposes the use of Abrysvo in pregnant women and strongly encourages the VRBPAC and FDA to reject the use of Abrysvo in pregnant women.
Sincerely,
Theresa Wrangham, Executive Director
References
1 American Lung Association. RSV Symptoms and Diagnosis. Mar. 7, 2023.
2 U.S. Centers for Disease Control and Prevention. Respiratory Syncytial Virus Infection (RSV). Oct. 28, 2022.
3 U.S. Centers for Disease Control and Prevention. RSV in Infants and Young Children. In: RSV Surveillance & Research. Oct. 27, 2022.
4 U.S. Centers for Disease Control and Prevention. RSV in Infants and Young Children. In: RSV References & Resources. Oct. 27, 2022.
5 U.S. Centers for Disease Control and Prevention. RSV in Infants and Young Children. In: RSV References & Resources. Oct. 27, 2022. 6 Breese CH, Weinberg GA, Iwane MK, et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N Engl J Med Feb. 5, 2009 E
Full-Letter: Download…
These people make me sick 🙁 :/
All we need now is little bit of fear campaing on TV over the dangers of RSV… The doctors will be riding along again. Anyone who question the new “cure” will be antivaxxer..
And who knows how many children will be again sacrificed at the altar of vaccines.
Disgusting.
Thank you David for your work! Study 329 book is one of my favorites. Very important and very sad book 🙁
recovery&renewal Retweeted
Kristina Kaiser
@AkathisiaRx
·
5h
Harming unborn #babies is just biz as usual for @US_FDA ‘s pay-to-play #drug approval system:
“For years, the FDA allowed a drug to be injected into pregnant women that was neither safe nor effective. Finally, it has been withdrawn.” https://tinyurl.com/yv6ukynmHarming
@MaryanneDemasi
FDA not fit for purpose – the Makena® fiasco
For years, the FDA allowed a drug to be injected into pregnant women that was neither safe, nor effective. Finally, it has been withdrawn.
MARYANNE DEMASI, PHD
16 MAY 2023
https://maryannedemasi.substack.com/p/fda-not-fit-for-purpose-the-makena?utm_source=post-email-title&publication_id=1044435&post_id=68715911&isFreemail=true&utm_medium=email
Previously, I wrote about Makena, a synthetic hormone given to millions of pregnant woman, to prevent premature birth. It was highly controversial because there was no robust proof that it was safe or effective, despite having FDA-approval.
Last month, after many years of use, the FDA finally decided to withdraw Makena from the market.
Adam Urato, a maternal-foetal medicine specialist at MetroWest Medical Centre, Massachusetts welcomed the decision, but said it took the FDA far too long.
“I cannot believe we’ve been injecting this hormone into pregnant women for 20 years, all the leading medical organisations recommended it, the FDA approved it – and it took this long to finally acknowledge the drug did not work,” he said.
Urato opposed the use of Makena from the start. He testified before the FDA, he wrote in the media and in medical journals, and helped petition the FDA to withdraw the drug from the market.
“crafting an approval,”
The decline of science at the FDA has become unmanageable
BMJ 2023; 381 doi: https://doi.org/10.1136/bmj.p1061 (Published 15 May 2023)
Cite this as: BMJ 2023;381:p1061
David B Ross, associate clinical professor of medicine
Author affiliations
rossdb@gwu.edu
The FDA’s legally enforceable regulations detail what “substantial evidence” and “adequate and well-controlled investigations” mean in greater depth, supplemented by guidelines to industry which, although not legally binding, explain the FDA’s current interpretation of drug manufacturers’ legal obligations.3 However imperfect, the FDA’s enforcement of these provisions assures prescribers, patients, and payers that effectiveness claims are based on science, not science fiction. A published FDA review showed efficacy deficiencies, in whole or in part, underlying initial rejection of 89 of 151 NDAs (59%), highlighting the continuing need for vigilance on this front.4
However, as Peter Doshi reports in The BMJ,5 the FDA subverted the legal standard for effectiveness in its 2019 approval of Recarbrio, a fixed dose combination of imipenem, cilastatin, and relebactam. While the FDA has previously approved products with marginal evidence of effectiveness,6 approval of the Recarbrio NDA was shocking given its lack of substantial evidence of effectiveness and the complete absence of adequate and well controlled clinical investigations on the actual indication of interest.
…….The Recarbrio approval is even more troubling because of other inexplicable departures from fundamental scientific, regulatory, and procedural principles. These included the failure to require “that each active component [of a fixed dose combination drug] contributes to the effect claimed for the product.”8 The FDA’s conclusion that Recarbrio at best does not reduce the efficacy of an approved drug can hardly be considered a demonstration that a component “contributes to the effect claimed for the product.” In addition, office directors responsible for approval decisions are required to provide “a rationale for concurrence or non-concurrence with the review team and the division director.”9 For Recarbrio, however, the “reviews” by both the office director and the division director responsible for its approval consist of nine words: “I concur with the review team’s assessment and recommendations.”
Finally, despite all the NDA’s defects, the FDA decided not to present it to an FDA advisory committee, as called for by law,10 on the basis of an astonishing statement that “there were no controversial issues that would benefit from advisory committee discussion.” The absence of adequate and well controlled clinical investigations in an NDA would normally cause the FDA to find the application to be unapprovable on its face and to refuse to even review it.11 Incredibly, the FDA granted Recarbrio a priority review, shortening the time to approval by 40%. Even more incredibly, the FDA designated Recarbrio as qualifying for financial incentives aimed at encouraging development of drugs to treat infections caused by resistant organisms—despite the lack of substantial evidence that Recarbrio does actually treat such infections.12
Scientific culture
What accounts for this descent into cargo cult science? Much of the blame must go to the FDA’s reliance on industry paid user fees. Over the past three decades the proportion of the FDA’s annual drug budget made up of such fees has risen from less than 10% (fiscal year 1994) to more than two thirds (fiscal year 2023).1314 In addition, the alluring “regulatory flexibilities” provided by the FDA Modernization Act of 1997 and the 21st Century Cures Act have become habit forming, enabling the FDA’s leadership and managers to deny scientific reality by defining effectiveness downward. In its quest to avoid difficult choices and hard decisions the FDA has increasingly embraced non-inferiority trials (or vice versa), ignoring the serious regulatory, clinical, and ethical problems caused by their misuse.15
However, the corruption of the FDA’s scientific culture remains the primary culprit driving the deterioration of safety and effectiveness standards. During my tenure at FDA, managers would admiringly speak of “crafting an approval,” as if it were a skilful demonstration of regulatory legerdemain rather than an act of scientific fabrication. The Recarbrio approval illustrates that the situation has, if anything, worsened since then. FDA leadership’s continued hostility towards meaningful peer review, transparency, and accountability dims the prospect for institutional self-renewal. So has the failure of much touted internal pathways for disagreement, which have amounted to little more than virtue signalling.16
Transparency
What can be done about this dismal situation? The first step is admitting that there’s a problem—that the decline of science at the FDA has become unmanageable. Fifteen years ago, in a trenchant essay, Peter Barton Hutt, chief counsel for the FDA from 1971 to 1975, wrote that “science at the [FDA] today is in a precarious position” and that “the agency is barely hanging on by its fingertips.”17 Warning that user fees had damaged FDA science and credibility while simultaneously disguising the damage, he called on Congress to adequately fund the FDA. Although politically fraught, tapering the FDA’s dependence on user fees would involve less than 0.2% of the annual federal budget. This would be a small price to pay for checking the continuing corrosion of the agency’s scientific integrity by user fees.
The second—and more achievable—step requires improving public access to the information received by the FDA, its reasoning, and its decisions. In addition to enabling meaningful peer review and engagement by providers, patients, researchers, healthcare organisations, and drug manufacturers with the FDA on the scientific basis for its actions, increased transparency would highlight the FDA’s value as a producer of information.18 Fifty years ago the agency issued regulations providing broad authority to disclose safety and effectiveness data.19 Subsequently, however, the FDA reinterpreted its authority under the Freedom of Information Act to significantly narrow the scope of information it would release.20 Its continuing refusal to disclose non-trade secret information, such as the effectiveness data in the Recarbrio NDA, is untenable given the FDA’s existing authorities21 and its ability to implement congressionally mandated transparency reforms, such as the requirement to post NDA action packages to the FDA’s website within 30 days of approval, without having to promulgate new regulations.10
The Recarbrio approval is a sentinel event, warning of a return to an era when drug effectiveness was an afterthought. Although the FDA crowed about this approval,12 it would have been better advised to remember that “for a successful technology, reality must take precedence over public relations, for nature cannot be fooled.”22
1 of 4
The Pharma Racket Writing on The Wall
Inbox
Josh Guetzkow from Jackanapes Junction
5:42 PM (2 hours ago)
Open in app or online
The Pharma Racket Writing on The Wall
Musings on a visit to the doctor
JOSH GUETZKOW
MAY 16
So there I am, sitting in the waiting room at a local health clinic in Tel Aviv, when out of the corner of my eye for a split second I spot the word “Pfizer” written on one of the screens they have dotting the walls:
Reads “Public Service. Sponsored by and on behalf of Pfizer which is solely responsible for the content.”
“Public Service,” it’s written. “Sponsored by and on behalf of Pfizer which is solely responsible for the content.”
What content? I had to wait a minute for the advertisement public service announcement generously paid for by Pfizer to come around again. It starts out with a startling warning: “Atrial fibrillation increases the risk of stroke by 5 times.”
Image
And then: “Do you have high blood pressure? With atrial fibrillation?”
Image
“What is your risk?”
Image
“Ask the medical staff today about treatment for high blood pressure and atrial fibrillation?”
Image
It will not have escaped readers of this blog that the CDC’s analysis of adverse event reports to its VAERS system for mRNA COVID vaccines found safety signals for atrial fibrillation, increased diastolic & systolic blood pressure and 26 types of stroke.
Image
Image
That’s in addition to a dozen or so published case series documenting onset of fibrillation and hypertension following COVID-19 vaccination — out of 3,400 published studies and reports on adverse events.
It should also not have escaped the reader’s attention that Pfizer has profited handsomely from sales of its blood thinning medication, Eliquis, which was the #5 best selling pharmaceutical in the world in 2021, with sales only increasing since then.
Image
Eliquis is used to prevent strokes from blood clots caused by atrial fibrillation. And they have a new drug, not yet available in Israel or most of the rest of the world outside the US, that is supposed to directly treat atrial fibrillation. Plus they make a few blood pressure medications as well (though they were hit with a scandal in 2022 when they had massive recalls due to a carcinogenic compound found in many lots).
So basically Pfizer creates the #1 selling pharmaceutical product in the world, the Comirnaty COVID vaccine, which apparently can cause atrial fibrillation and high blood pressure. It then turns around and pays for advertisements public service announcements to increase sales of its products that are used to treat the problems caused by its vaccine. (Direct to consumer pharma ads are not allowed in Israel.)
Or as my colleague Professor Retsef Levi wrote on Twitter: “Pfizer buys the country, sells it a vaccine that causes high blood pressure, stroke, atrial fibrillation and other cardiac problems, then markets its drugs through the largest HMO in the country.”
Ladies and gentlemen, what we have here is a classic case of a racket, in the traditional meaning of “an organized criminal act in which the perpetrators…offer a service that solves a problem that would not exist without the racket.”¹
Nothing new, I know. Such is the nature of the pharmaceutical business model. For that matter, is there any major industry in the modern world that doesn’t work the same way?
See here for possible etymology of this term: “English pickpockets, once the best of the breed, invented the ploy of creating disturbances in the street to distract their victims while they emptied their pockets. This practice was so common that a law was passed in 1697 forbidding the throwing of firecrackers and other devices causing a racket on the city streets.”
Prof Norman Fenton
@profnfenton
·
1. Within 60 seconds of me uploading this 2-minute clip about the AstraZeneca vaccine (from my testimony to the Corona Investigative Committee, Germany) YouTube removed it and gave me a strike:
Prof Norman Fenton
@profnfenton
·
3. Perhaps if us “conspiracy theorists” with our “bizarre claims” (early 2021) the vaxx could kill hadn’t been censored, while claims the vaxx was 100% effective were everywhere, then people like Lisa Shaw, Michelle Barlow, Zion, & Stephen Wright would still be alive today.
https://wherearethenumbers.substack.com/p/when-real-misinformation-kills?utm_source=substack&utm_medium=email
Prof Norman Fenton Retweeted
Josh Guetzkow
@joshg99
·
Was the Pfizer/BioNTech vaccine clinical trial a bait-and-switch? There were >44,000 people in the trial, but only ~250 of them were given doses made with a new manufacturing method (‘process 2’) that was used to make enough doses to sell around the world.
https://twitter.com/joshg99/status/1658421192326365185
Josh Guetzkow
@joshg99
·
To our knowledge, the safety and efficacy comparison they planned to do with those 250 subjects has never been published and has not been released in the FOIA’d documents that Pfizer submitted to the FDA. Was the comparison ever done? Where are the results?
Brook Jackson
@IamBrookJackson
Unacceptable! Wow! ATTN SHERIFFS: You have the ability to stop these shots from injuring or killing more! Ask me how.
Josh Guetzkow
@joshg99
·
Replying to @IamBrookJackson and @KenPaxtonTX
Our initial analysis indicate that the subjects who got the process 2 doses had 2x-2.5x the rate of adverse events compared to original recipe Comirnaty. We’d love to get discovery because we have A LOT of questions for Pfizer.
https://twitter.com/joshg99/status/1658456295580786689
“So much for the 100% efficacy” …
“Pfizer pledged to closely track the vaccine’s real-world use for more evidence. Ultimately the advisers unanimously decided that the shot is effective — and voted 10-4 that there’s adequate safety data. The FDA will consider Thursday’s recommendations in making the final decision on approval.”
“If you’re in any sense risking premature births with this vaccine, I think there will be a big price to pay,” said Dr. Paul Offit of the Children’s Hospital of Philadelphia, among the panelists who voted “no” on the safety question.
If you’re pregnant, you can now get a vaccine that protects you and your child from a dangerous respiratory virus
BYLAURAN NEERGAARD AND THE ASSOCIATED PRESS
May 19, 2023 at 12:18 AM GMT+1
https://fortune.com/well/2023/05/18/pfizer-fda-approves-rsv-vaccine-pregnancy-respiratory-virus/
A first-of-its-kind RSV vaccine for pregnant women guards their newborns against the scary respiratory virus — and federal health advisers on Thursday backed Pfizer’s shot despite some lingering questions.
RSV fills hospitals with wheezing babies each fall and winter, and the virus struck earlier than usual and especially hard in the U.S. this past year.
If the vaccine pans out, “many infants and their parents will breathe easier in the coming years,” said Dr. Jay Portnoy, a member of the Food and Drug Administration advisory panel from Children’s Mercy Hospital in Kansas City, Missouri.
The idea: Give women a single injection late in pregnancy, between 24 weeks and 36 weeks, so they develop RSV-fighting antibodies that pass through the placenta — just like they pass protection against other bugs to their babies.
In Pfizer’s international study of nearly 7,400 pregnant women, maternal vaccination proved 82% effective at preventing severe RSV during babies’ most vulnerable first three months of life. At age 6 months, it still was proving 69% protective against severe illness.
Pfizer said there were no signs of safety problems but the FDA did ask its scientific advisers to consider whether a slight difference in premature birth between vaccinated moms and those given a dummy shot was of concern. Debate over whether that was really a hint of trouble or just due to chance dominated the panel’s daylong meeting.
Pfizer pledged to closely track the vaccine’s real-world use for more evidence. Ultimately the advisers unanimously decided that the shot is effective — and voted 10-4 that there’s adequate safety data. The FDA will consider Thursday’s recommendations in making the final decision on approval.
“If you’re in any sense risking premature births with this vaccine, I think there will be a big price to pay,” said Dr. Paul Offit of the Children’s Hospital of Philadelphia, among the panelists who voted “no” on the safety question.
If the FDA ultimately approves the maternal shot, it would mark a second milestone in the decades-long quest to prevent the respiratory syncytial virus. Earlier this month the FDA approved the world’s first RSV vaccine, rival GSK’s shot for older adults, who also are at high risk. There isn’t a vaccine yet for children, but Pfizer is about to begin testing one.
Here are some things to know:
What happens next?
FDA’s advisers already have recommended approving Pfizer’s vaccine for older adults, and the agency is expected to make a decision by month’s end. Whether to use the same shot in pregnant women will be a separate FDA decision, expected in August.