
April is the cruellest month,
breeding Lilacs out of the dead land,
mixing Memory and desire,
stirring Dull roots with spring rain.
T.S. Eliot: The Waste-Land
In August 1914, the First World War took shape. In August 1939, the Second World War was in preparation with Germany launching the first attacks on September 1.
At the end of July 2020, the first patient in the US was were recruited to the Pfizer – ICON trial. It really got going in August. On August 6, the first patient was recruited in Buenos Aires., with 9 others that day.
This post follows on from Vaccine Rag and Vaccine Tango as well as Two to Tango.
Dose 1
Augusto Roux had his first vaccine dose at 6.50 PM on August 21, three days after his 36th birthday. Younger people were being seen in the afternoon with older individuals in the mornings.
The photo above captures the scene. Travel to and from the clinic was by Taxi. At the height of the epidemic, many were very cautious, wearing as a comprehensive a set of masks as we could find. Augusto was particularly concerned about causing problems for his mother, who was in an at risk group.
Augusto had pain at the injection site, with pale feces and dark urine for four days afterwards. He was intolerant of fatty foods. Putting all this together, he figured he had a liver problem. He contacted the research team by phone on August 23, who classified his difficulties as Toxicity Grade 1 – see Disappeared in Argentina.
The trial monitors seem to have viewed the problem primarily as pain at the injection site. They took care to rule out any error in the way the vaccine was administered.
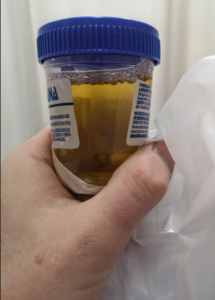
This urine sample was taken a few days after Dose 2, when the coca-cola effect was wearing off.
Everyone was being treated virtually in Argentina just like in North America and Europe. Augusto’s family doctor suggested a liver scan but denied that his problems were likely to be linked to the vaccine.
Dose 2
We now know Augusto had received the active vaccine.
He had Dose 2 on September 9. Several hours later he became violently ill with coca-cola colored urine, fever, breathlessness, chest pain, and other problems. He was out of action, mostly unconscious for nearly three days. At one point he collapsed on his bathroom floor.
When able to, he made his way to the Hospital Alemán, where he was admitted for three days. His investigations show pericarditis. His view was that he had a liver problem. His liver function was not investigated thoroughly perhaps because he was not obviously jaundiced. The tests showed mildly raised hepatic (liver) enzymes. These kept rising after he left hospital. To this day he has difficulties with fatty food.
He was discharged from Alemán on September 14 with a diagnosis of an adverse reaction to the Pfizer vaccine. But in the Pfizer/ICON research record, based at the Hospital Militar, he was listed as Suspected COVID 19.
He remained unwell and worried at home. On September 22, some blood test results, which he had done privately, came back. His liver enzymes had doubled – see Disappeared in Argentina for the results. They were now distinctly high.
He contacted the Hospital Alemán, asking about his liver, who responded by email saying several things that didn’t help.
They congratulate him on being well and say his liver tests were normal (which they almost were at the time of discharge – but his results now are inconsistent with that). They can’t run tests on him or help because this will interfere with the vaccine research – it would involve breaking the blind. He finds he cannot download the tests they have done on him from the hospital portal. It crashes.
In an email, Alemán also say: “We spoke with the Hospital Militar’s research team to inform them of your case, as well as that of other patients we had last week, our job is to respect and not compromise the study in which you are voluntarily participating. We have been instructed not to intervene as far as antibody monitoring is concerned. I would ask you to communicate with the relevant investigator”.
His medical friends tell him he has a problem and they can’t believe how he is being treated. There is scope here for things to fall apart.
Hanging on the Telephone
On September 23, Augusto calls the research team. The consent form he signed opened this door to medical care if he was unwell. But the picture is rapidly getting more complicated. He now needs to know what is wrong with him but also whether the vaccine caused it?
He also needs a letter signed for work, as he had been too ill to return there and would require medical certification of absence. He is told the doctors will call him.
He had a phone call close to midnight from Diego Wappner, who had just come off his shift running the research clinic, which at this point was recruiting over 250 people per day. They had talked once before when Augusto was in hospital, when Wappner got the impression Augusto was admitted for Coronavirus.
Augusto’s email from Alemán suggests they were being told what to do by the Research Team. Wappner has heard about this and is mystified. He wonders if Alemán have been contacted by the military.
Augusto says he went to hospital to have his liver investigated but Alemán put him on a COVID protocol without even taking steps to minimise the spread of infection. This was unacceptable. Augusto’s brother has liver disease – if Augusto’s reaction is caused by the vaccine what if this reaction happened to his brother? What about Augusto’s mother who has respiratory problems?
Wappner repeatedly assures Augusto he has a case and says that he will get Polack to call him and straighten things out.
Diego Wappner is on the right in this photo with Fernando Polack in the middle.

Boss of Bosses
The following day, again late at night, Augusto got a phone call from Fernando Polack.
Polack seems sympathetic. He seems to think Augusto’s doctors should be able to help him and should be willing to do tests to diagnose his liver condition. Augusto tells him he is not a doctor and has no idea what tests would be appropriate but he does know something is wrong and it is not right that he is not being looked after.
Why is he being tied up in bureaucratic madness? Polack says there is nothing fishy going on but the research team cannot do anything to make a diagnosis, run tests or offer treatment.
He says there is a lot of confusion in the research because they are dealing with so many cases and perhaps messages have got crossed. Another patient’s notes did end up being mixed up in Augusto’s record, so it is possible the wrong email was sent to the wrong person – See Welcome to Mondor. But it doesn’t look like this is what is happening here.
Augusto mentions the Research App is useless – it only lets him record problems as COVID. It does not let him link problems to the vaccine. [If he goes blind after the vaccine, which the vaccine can cause, he could not record this but he could record loss of taste or smell – well known features of COVID].
Polack says this is because reactions to the vaccine are rare and predictable – they do not include unusual events like Augusto’s which, if linked to the vaccine, are exceedingly rare. They’ve recruited tens of thousands of people and seen nothing like this. [This App could easily have had a free text spot for unusual events].
Augusto chases the topic of links between the Hospital Alemán and Militar. Polack says there are none except they have to call if someone in the trial is admitted to any hospital to get the details of any tests and diagnoses.
Augusto makes it clear that the trial broke down when he was sick after the second dose. No-one contacted him to find out if he was alive or dead or to organize care of him if he was having problems. At this point, he needs to know if he got the active vaccine.
Polack says they cannot break the trial blind to tell him this. They can only break it if he is at risk of dying. Augusto asks to be shown the form and the part of the protocol that says this.
If the research team cannot do this and cannot break the blind in his case, Augusto wants this written down to show at work and he indicates he will be taking the matter up legally.
Forked Tongues

On August 23, the day Augusto reported his Dose 1 reactions to the research team, 287 volunteers were injected with Dose 1 in the Hospital Militar. The first 102 went fine. Something then happened in late morning, perhaps after lunch, starting with volunteer 103 that day.
Of those vaccinated after that, 52 ended up being unblinded on August 31. Of those who were unblinded, 31 did not have Dose 2 of the vaccine. Despite refusing the second dose, some of these volunteers opted to stay in the trial for monitoring purposes. Some of those not taking Dose 2 are recorded as opting to stay in and others are not recorded as opting to stay in but appear to have stayed in. Others are clearly recorded as not opting to stay in.
Twenty-three (23) stayed in the research and opted to have the second vaccine. Four of these had significant adverse events, after the second dose.
While something was happening to the 52, who were later unblinded, another 39 were getting the correct dose or at least were not told of any problems and were not unblinded on August 31.
Of the 52 unblinded, in one document the reason given is a mix up in the dose, but in the same document all are recorded in the randomization log as being randomized to Vaccine and as having been given the correct dose for Dose 1. In other files a wrong dose is listed or a wrong product. Three different Pfizer files give three different explanations for what happened – none of which make sense.
There is nothing like this at any other centre in the trial – few if any other centres were processing 52 people per day.
There were other strange events on August 23. The volunteers that day contributed 3 cases of COVID after placebo to the 170 cases that provided the basis for FDA to approve this vaccine under Emergency Use Authorization. The NAB test was negative in two of these and Not Done in the third – so the most specific test did not support a diagnosis of COVID in these three. See Two to Tango.
August 31 was the last day on which Site 1231 recruited new patients, Three weeks later it re-opened as Site 4444. The sole positive COVID case after vaccine from Buenos Aires in the 170 cases came from the 4444 group. Without this, the vaccine would have had 100% efficacy in Buenos Aires.
So it makes some sense that Site 1231 took stock on August 31 and for some reason opted to unblind 52 subjects from August 23. Unblinding is what was recorded but what did this mean? Everyone administering the dose appears to have known who was getting what – the placebo and vaccine looked different.
The only people who were blind were the volunteers but they only seem to have been told two weeks later.
Around the time Augusto is in Alemán, the researchers appear to have told some people coming for Dose 2 that something has gone wrong, and presumably tell them they have had the active vaccine.
A week later, Polack tells Augusto there is no way they can break the blind in any case unless the person is dying. Augusto says this is crazy and asks for this statement in writing. Polack backs down – maybe they could break the blind if a person is very ill. Augusto asks for that in writing but never gets it.
He gets nothing. He makes a complaint to the Argentine Regulator, ANMAT. Polack then writes his version of their conversation in the research record and in Augusto’s medical record and states that Augusto has a constitutional anxiety toppling into paranoia – in other words that he is mentally ill.
Despite what Polack says in their conversation, there are now good grounds to think these mRNA vaccines can cause liver and gall-bladder problems – see Tales of the Unexpected.
It is easy to see in this sequence of events how wires might have gotten crossed. Augusto had a set of problems after the second dose of vaccine that were clearly linked to the vaccine, but the system from the COVID trial app to the information given to companies like iTrials was close to useless. This study was happening on a wing and prayer without detailed studies on the possible problems beside local vaccine reactogenicity that might arise.
The failure of investigators like Wappner and Polack to pay any heed to things happening to volunteers like Augusto meant the trials were written up as demonstrating the vaccines were astonishingly effective and very safe and publications claiming this in the New England Journal of Misinformation were then used to dismiss people who were injured.
An atmosphere was created where doctors were likely to lose jobs for recognizing obvious problems. The injured ended up being treated as contagious and were shunned like lepers. It wasn’t their injuries that were contagious. Their existence, which testified to the reality of harms, was contagious
There is scope for crossed wires in these events – but what’s up with Polack unblinding 52 people at one point and then telling Augusto a short while later that there is no way they can unblind him?

I’m a Celebrity – GMOOH…
What did COVID-19 leave us?
Diego Wappner
Infant Foundation
August 23, 2021, the FDA granted full approval to the mRNA vaccine
https://www.researchgate.net/publication/357610741_Que_nos_dejo_el_COVID-19
Clinical trials showed no serious adverse events or safety within 8
weeks after vaccination, an important indicator of developmental quality
achieved through the different actors.
The global effort almost two years after the start of the COVID pandemic-
19 has resulted in the development and distribution of safe and effective vaccines endorsed by the competent health authorities.
Undoubtedly, we are living in a time of great change, this unexpected situation.
Juan Gérvas Retweeted
Joan-Ramon Laporte
@joanrlaporte
Negació del debat científic. Inquisició contra ciència. El Professor Domingo forçat a dimitir com a director de revista científica per haver publicat un article que posava en dubte la seguretat de les vacunes mRNA
Editor-in-Chief of Renowned Science Journal Ousted for Publishing Science Questioning COVID-19 Vaccine Safety
https://www.theepochtimes.com/health/editor-in-chief-of-renowned-science-journal-ousted-for-publishing-science-questioning-covid-19-vaccine-safety_4830112.html?utm_campaign=socialshare_twitter&utm_source=twitter.com
However, Domingo said that the journal has already picked a successor for his position—someone with clear ties to the pharmaceutical industry: Bryan Delaney, Ph.D.
According to his LinkedIn page, Delaney is a toxicologist who currently works for Haleon. Haleon is pharmaceutical giant GlaxoSmithKlein’s new brand name for its consumer health unit.
Since the beginning of 2022, we have seen many scientists and medical doctors risking their careers, their medical licenses, and even their personal safety to fight for scientific freedom and integrity.
Dr Aseem Malhotra
@DrAseemMalhotra
If you want to know why many journalists including at the BBC (I have many friends there) have themselves been unwittingly duped by Big Pharma narrative you must read this.Why are journalists not fact checking an industry whose business model is fraud ?THAT is the real question!
Paul D. Thacker
@thackerpd
BBC Should Step Aside and Allow the Biopharmaceutical Industry to Do its Own Public Relations https://disinformationchronicle.substack.com/p/bbc-should-step-aside-and-allow-the… With so many prior screw ups, now is the time for Big Fact Check to shut down.
https://disinformationchronicle.substack.com/p/bbc-should-step-aside-and-allow-the
And when governments first began facing the possibility of a global pandemic, who did the world come to rely on to create the vaccines in the United States—Moncef Slaoui, a GlaxoSmithKline executive, who I caught a decade ago misrepresenting scientific research on the safety of a diabetes drug that harmed tens of thousands of people.
“read like a spy novel.”
https://www.thedailybeast.com/the-sketchy-past-of-moncef-slaoui-trumps-coronavirus-vaccine-czar
I’m a Celebrity – Matt Hancock – promotes his new book – Covid Diaries – in the jungle
About the Author – He retains all his fingers.
https://www.amazon.co.uk/Pandemic-Diaries-inside-Britains-against/dp/1785907743
I’m a Celebrity – GSK is an advertiser in the ad breaks – DTCA – Shingles Vaccine – in the jungle
Fernando Polack and Diego Wappner – pulling-fast-ones – on Augusto – in the jungle…
A huge THANK YOU to Augusto Roux! Speaking out can’t be easy in these circumstances, but it may end up doing a world of good.
Not sure if this is obvious already – but perhaps Site #4444, the miraculous “second wave” of Pfizer’s Buenos Aires vaccine trial, represented a Change of Venue?
The picture of trial subjects being picked up by taxi, double-masked and wearing face shields and other fancy protective gear, is telling. If Argentina was at all like the USA in August 2020, lots of folks were jumping at the chance to get into vaccine trials. Those who succeeded may often have been middle-class and up, with excellent Wi-Fi and likely other “connections” as well (just like those who later jumped the queue to get the first scarce vaccine doses).
For Pfizer, this could be a problem. These people could protect themselves pretty well from the pandemic. They had good medical care; even more important, they could work from home and have their food and supplies delivered. As a result there were probably very few cases of Covid-19, even in the placebo group. Wouldn’t that make it awfully hard to demonstrate the protective qualities of the vaccine?
A second problem: Middle-class subjects with even modest “connections” (along with a bit of education and computer-savvy) were more likely to get noticed if they complained about adverse events. They might generate too much noise.
Better to switch to a more typical subject pool: working-class people with few choices about their health-care, and no doctors or professors in their circle of friends. And plenty of exposure to the virus, at work, on public transit and in crowded apartment blocks.
The sheer number of doctors employed on this trial in Argentina suggests they could not all have been working at the Hospital Militar. Do we know what other locations were being used, and what was happening there? That might help unravel the sudden success in coming up with the required 170 cases of Covid-19 – and a lot else besides.
When Augusto realised for sure he was being sacrificed by Pollack and collaborators his anxiety surely must have been sky high. It would be for anybody (as those who have been shafted by medics and their allies in other horrendous situations know) .It’s the heart sink realisation that we live in a world where corruption amongst those responsible for health and well being is not just a minor aberration amongst a few rogues but is rife and lives of the most vulnerable are dispensable. Right now we have no idea how many have been silently sacrificed by the actions of the pollacks and co- conspirators. It has taken a long time before more of those in positions to tell the truth have felt able to join forces with those who have been speaking out . Whether this learning about compliance could be transferable to all areas of corruption in the medical world is debatable. Respect to you and your allies Augusto.
1 of 2
How Long Did the Pfizer COVID Vaccine Trial Last?
Inbox
Josh Guetzkow from Jackanapes Junction
8:55 AM (3 hours ago)
Open in app or online
How Long Did the Pfizer COVID Vaccine Trial Last?
Let’s just say, they should have used Pfizer’s little blue pill.
JOSH GUETZKOW
NOV 15
Second time’s the charm? Pfizer tries again with OTC Viagra in the U.K. | Fierce Pharma
How long did the Pfizer/BioNTech COVID vaccine clinical trial last?
The short answer is: 97 days.
Here’s the long answer:
When unblinding began in earnest on December 14, 2020 — with the FDA’s blessing 3 days after they granted an EUA — the trial subjects in the Phase 2/3 trial for ages 16+ had been in the trial on average for 97 days. That’s 3 months plus a week.
I arrived at this figure by analyzing the Pfizer/BioNTech clinical trial data released via court order, specifically using the file at this link. The figure below shows the pace of unblinding across treatment arms from Dec 14 – Mar 13. (March 13 was the data cutoff date for Pfizer’s FDA biological license application for full approval, so any trial data after that is not included in the court order.)
Image
The first subjects recruited to phase 2/3 were given their first dose on July 27, 2020. From then to Dec. 14 is 140 days, or over 4 1/2 months. But many subjects were recruited long after July 27. In fact, 19 subjects had their first dose after Dec. 14!
But hold on a second — they weren’t all unblinded on Dec 14. Shouldn’t we count how long each person was in the trial before being unblinded? If you count that way, the average subject was in the trial for 137 days before unblinding.¹
Except the only way that could make sense is if the unblinding was random. If it wasn’t, then the moment you start unblinding, you can’t calculate an unbiased treatment effect by comparing treatment and control groups — in other words, the conditions necessary for a Randomized Controlled Trial (RCT) no longer hold.
And indeed, the unblinding was not random: The Pfizer trial unblinded younger and older volunteers at different rates – not randomly!
Image
This means the trial effectively ended on Dec 14, 2020, and we’re back where we started: Subjects were in the trial an average of 97 days.
To put a little more meat on this: in the dataset linked above, there are 22,015 placebo subjects and 22,024 subjects in the treatment group. By the March 13 cutoff, 20,935 of the placebo subjects had been unblinded, with some 19,605 of them (89%) having received their first real dose of the vaccine before the data cutoff — on average 6 days after unblinding. Of those in the treatment group, 20,400 had been unblinded by the data cutoff date.
There were actually 19 subjects recruited after December 14, with the last one receiving the first dose on Jan. 8. If we set those aside, the minimum number of days in trial prior to Dec 14 was 3 days and maximum was 120, with a standard deviation of 25 days.
Once the trial effectively ended on Dec. 14, accurate assessment the safety of the vaccine across treatment and control groups was no longer possible—especially since the placebo subjects started receiving their first real dose of the vaccine very soon after unblinding.
There were 39 placebo subjects from Phase 1 who may have been grandfathered into the 2/3 trial, this analysis excludes them. My tweet about this from a couple days ago and Jason Hart’s post about it included those subjects, which accounts for any discrepancy between this post and those. Also in my tweet, I didn’t take the space to mention the subjects who had their first dose after Dec. 14.
1 of 1
Swissmedic and Vaccinating Doctors Criminally Sued in Switzerland for Authorizing and Administering Covid-19 mRNA …
Inbox
Josh Guetzkow from Jackanapes Junction Unsubscribe
7:14 AM (4 hours ago)
Jackanapes Junction cross-posted a post from Live to Fight Another Day
Josh Guetzkow
Nov 15 · Jackanapes Junction
I had the honor to meet the lawyer behind this case, Philipp Kruse, in Vienna in September. Such a gentleman but also an indefatigable warrior. Good luck to him!
Swissmedic and Vaccinating Doctors Criminally Sued in Switzerland for Authorizing and Administering Covid-19 mRNA Jabs
Do not underestimate the Swiss diligence.
ANDREAS OEHLER
NOV 15
SAVE
The detail-oriented Swiss are taking their medical establishment to task in a big way for authorizing and administering the Covid-19 mRNA jabs, in a formal criminal complaint.
“That is what the criminal complaint against Swissmedic is about” (SRF, 2022.11.14):
That’s what it’s all about: On July 14, 2022, a lawyer submitted a 300-page criminal complaint to the responsible cantonal public prosecutor’s office on behalf of six people allegedly injured by mRNA vaccinations. It is directed against three representatives of the Swiss licensing and supervisory authority for medicinal products and medical devices (Swissmedic) and five vaccinating doctors from the Inselspital in Bern. A criminal investigation is to be opened against them. The lawyer has now gone public with a media conference.
These are the plaintiffs: The lawyer for those affected, Philipp Kruse, is a declared opponent of vaccination and Covid measures. He represented people who refused to wear masks or parents who didn’t want their children to take part in pool tests. Doctors who were noticed as corona skeptics also appeared at the media conference.
This is what the indictment says: The defendants are accused of violating basic drug law due diligence by allowing and administering the Covid 19 vaccination. There are a number of other charges listed, including intentional or possibly negligent bodily harm, endangering life, killing and abortion.
These are the alleged damages: According to lawyer Kruse, the damages range from circular hair loss, derailment of the menstrual cycle to polyarthritis, muscle weakness and chronic exhaustion to the death of a 20-year-old person. Some of the six victims listed are still unable to work. The connection to the Covid 19 vaccination was confirmed by experts in five cases. In the case of the deceased, the causal connection must be proven on the basis of pathological examinations. However, these investigations are not yet complete.
That’s what Swissmedic says: Nothing. Swissmedic does not want to comment on the ongoing court proceedings. The Federal Office of Public Health and the Federal Vaccination Commission also do not want to comment.
What may fly under the radar in other countries, in terms of the lack of accountability and responsibility for Covid-19 jabbing injuries, is unlikely to pass muster in Switzerland. The Swiss proud themselves on being OBJECTIVE, diligent, thorough and just. Because of these very high public expectations, it is impossible to sweep under the carpet, gaslight, or outright ignore the laws on the books over there, let’s hope:
It is about these articles of the Medicines Act
Art. 3 Duty of care 1 Anyone who handles medicinal products must take all the necessary measures based on the current state of science and technology to ensure that the health of humans and animals is not endangered.
Chapter 8: Penal Provisions Art. 86233 Crimes and misdemeanors 1 Anyone who willfully: a. manufactures, places on the market, uses, prescribes medicinal products without the necessary authorization or authorization, contrary to the terms and conditions associated with an authorization or authorization or contrary to the due diligence obligations stipulated in Articles 3, 7, 21, 22, 26, 29 and 42, imports, exports or trades abroad; (…).
Link to the Medicines Act .
Also of note here is that Swissmedic is the key conduit of the global vaccination programmes in partnership with Bill&Melinda Gates Foundation and WHO, and also FDA.
Good luck in court!
Tracking – Augusto…
Pfizer and Moderna launch trials to track whether health issues arise YEARS after getting their Covid vaccines
Moderna and Pfizer will investigate whether their shots cause long term issues
The firms will track people who suffered adverse affects from the shots
They will attempt to determine the long term negative effects they have had
Heart inflammation has been the most reported severe side effect of the vaccine
https://www.dailymail.co.uk/health/article-11426007/Pfizer-Moderna-launch-trials-track-issues-arise-YEARS-getting-vaccines.html
Pfizer and Moderna have launched trials to determine whether there are any long-term negative health impacts associated with their Covid vaccines.
The studies will involve monitoring the small number of Americans who suffered rare side effects after receiving the shots over the past two years.
Both firms are required to carry out this long-term research by the Food and Drug Administration (FDA) as a condition of approval earlier this year.
Inflammation of the heart has been the most common serious adverse effect reported from the shots – though it is still very rare.
A study by British Columbia Centre for Disease Control, in Canada, found that 58 of every million recipients of Moderna’s two-shot vaccine developed the condition.
The same study found that 21 of every million recipients of the original two-dose Pfizer vaccine also suffered the heart issue.
Cases were most common among men under the age of 30, affecting more than 250 per every one million men aged 18 to 29.
The Centers for Disease Control and Prevention (CDC) said it has recorded aronud 1,000 cases of heart inflammation among under-18s who received Covid shots.
While these cases usually resolve themselves without medical intervention, some fear there could be long term heart damage.
Moderna, based in Cambridge, Massachusetts, has already launched two trials tracking its shot’s adverse affects, the most recent in September.
New York City’s Pfizer told NBC it it will launch a trial in the coming months that includes 500 teens and young adults under the age of 21.
The firms will follow some patients for up to five years, but results are expected to start coming in as early as next year, NBC reports.
Both companies used mRNA technology to develop their shots.
While mRNA has existed for decades, the Covid vaccines were the first to use the technology to develop a medical product used at this scale.
Experts believe mRNA is perfectly safe for use, though the little precedent in using it for other medication left many American uneasy about the shots.
Billions of doses of mRNA vaccines have now been administered worldwide, with very few side effects reported.
Myocarditis – inflammation of the heart muscles – is the most commonly reported severe vaccine-related adverse effect.
The condition occurs when the middle layer of the heart wall becomes inflamed.
Sufferers may feel shortness of breath, fatigue and chest pain – among other symptoms.
It usually resolves on its own but in rare cases it can lead to a heart attack, stroke or even death.
Pericarditis occurs when the pericardium, the outer layer of heart tissue, becomes swollen.
It has similar symptoms to myocarditis, and is also a relatively mild condition that will often resolve itself.
Both conditions are known to appear in people who had recently suffered a viral infection like Covid or the flu.
Some studies have suggested Covid is significantly more likely to cause myocarditis than the shots themselves.
A study published last week in the Journal of the American College of Cardiology found that rates of the conditions after vaccination were most common in younger people.
The concern about young people – who are less likely to get severe Covid – has led to some countries limiting the shot for certain age groups.
Denmark and Norway have already banned Covid vaccines for non-seniors, while Sweden has stopped recommending them for 12 to 17-year-olds.
Da’Vion Miller, 29, of Detroit, Michigan, began suffering fatigue, shortness of breath and dizziness two days after receiving the first dose of the Pfizer vaccine last October.
He was rushed to the hospital, where he was diagnosed with both myocarditis and pericarditis.
Doctors advised Mr Miller against receiving a second shot of the Covid vaccine, NBC reports.
Isaiah Harris, who was 18 at the time, of Springfield, Arkansas, also suffered a case of vaccine onset myocarditis last year.
He told 4029 News: ‘I was actually driving down the interstate, and all of a sudden, my heart rate just went way up, and it felt like it was beating out of my chest… I woke up the next morning and could barely breathe.’
The teen was rushed to a local emergency room, where doctors determined that he was suffering a heart attack caused by myocarditis.
Only a day earlier he had received a second dose of the Pfizer COVID-19 vaccine.
A report published in February this year tied the deaths of two teens, one in Connecticut and one from Michigan, to the Pfizer COVID-19 vaccine as well.
The pair each died within a week of receiving the shot.
Scientists determined that they suffered toxic cardiomyopathy caused by an immune response to the vaccine.
The condition occurs when a person’s heart muscle can no longer properly pump blood across the body.
Florida Surgeon General Dr Joseph Ladapo last month recommended against people under 40 getting the shots.
However, the research paper he used to justify the move was widely denounced by scientists as flawed.
The long-term safety trials carried out by Moderna and Pfizer will be the first to determine the true risk of receiving the mRNA COVID-19 vaccines.
The shots were a novel product that first became available in late 2020 on an emergency authorization approval.
While the firms went through rigorous testing to get approval for their shots, long term data is still unavailable as the earliest recipients are still only two years removed from getting vaccinated.
Robert F. Kennedy Jr
@RobertKennedyJr
·
24m
Listen to my latest podcast with Peter McCullough
https://anchor.fm/rfkjr/episodes/Censoring-Dr-Peter-McCullough-e1qn73p
“this is a warning to anyone who speaks up” – Dr. P. McCullough
Edward Dowd
@EdwardDowd
https://vigilantfox.substack.com/p/something-horrible-is-going-on-the
Something horrible is going on…
Brook Jackson
@IamBrookJackson
It’s a good day when the attorney that fired you for wanting to inform the public that Pfizer’s vaccine is a FRAUD is no longer representing Pfizer.
https://twitter.com/IamBrookJackson/status/1593038268752273408
EXCEPTIONAL Full-Length Feature :
BMJ INVESTIGATION
FDA oversight of clinical trials is “grossly inadequate,” say experts Covid-19 vaccines and drugs were developed at “warp speed” and now experts are concerned that the US Food and Drug Administration inspected too few clinical trial sites. Maryanne Demasi reports
http://press.psprings.co.uk/bmj/november/FDAoversight.pdf
A former staffer in the FDA’s Office of Criminal Investigations was also concerned aboutthe agency’s failure to fully tackle Jackson’s complaint about falsified data in Pfizer’s mRNA vaccine trial. In an email dated March 2021,they wrote, “Having worked at the FDA, I see it as surprising, for many reasons, that the agency turned a blind eye . . . They likely feared the criticism they undoubtedly would have received for holding up a vaccine (which they knew they would eventually approve anyway) at the expense of untold lives lost.”
The former FDA employee, who signed a non-disclosure agreement and did not respond to interview requests, went on to write, “My point here is that instead of the regulators protecting the public, they were complicit. At the time, they may have been doing what they believed to be the right thing under extraordinary circumstances.But now,they may soon have some explaining to do.”
Aseem Malhotra Retweeted
RougeNoir
@RougeNoirUK
What a lovely interview with @beverleyturner & John Campbell on @GBNEWS
https://youtu.be/0LWx8d0p158
“It’s the breach of trust” – John Campbell
Josh Guetzkow Retweeted
Adam Rowland MSc
@oneadds
The Questions i submitted at the MHRA board meeting this morning. Guess what… they decided against answering any of them. Dame June Raine even laughed at one question a vaccine injured person posted.
@MHRAgovuk @MHRAmedicines @10DowningStreet @PSCommissioner
https://twitter.com/oneadds/status/1592596122752909317
Josh Guetzkow
@joshg99
5 Israeli teenagers have died of sudden cardiac death in the last 72 hours.
Further to the above-mentioned Adam Rowland, please read this linked article:-
http://www.wharfedaleclinic.co.uk/our-message-of-support-to-adam-rowland-and-a-call-to-other-healthcare-professionals/
Adam, a member of our AD Withdrawal Group, has suffered unprecedented poor health over the past couple of years.
He joined our group suffering protracted withdrawal from a series of different SSRI drugs. He had lost everything in life that was dear to him and was having a difficult time coming to terms with all that had happened.
Through our group, I do offer individual, email support for any member in need of more than that which our zoom meetings can offer. In this way, over many months, I have got to know Adam very, very well. His suffering has been horrific – not least due to the fact that, at every turn, he was being told that it was all “in his head”.
Over the first weeks of contact with Adam, it became obvious that there was a lot more going on with him than protracted withdrawal alone, even as horrid as that can be in its own rights. We encouraged him to contact his GP and to get answers for his physical symptoms. After many visits to A&E, Adam was eventually admitted to hospital where it was found that he had many blood clots and extra concerns were raised about his heart. Again, he was repeatedly told that most of the problems remained “in his head”.
Due to his background in Sports Therapy etc., Adam knew exactly what the tests were that needed to be carried out in order to detect the real extent of his problems. All fell on deaf ears at his local hospital.
Adam fought to be admitted to a specialist unit at a London hospital for the real assessments of need to take place. He managed to get this done on the NHS after much wrangling of the authorities.
Adam’s problems are Covid vaccine related; that has now been established. He needs many further tests and biopsies, as indicated by the London Consultants, but is still awaiting these in his local area.
At the moment, Adam is back in his local hospital having been admitted through A&E with severe chest pains etc. He is determined to have all the recommended tests done – either locally or in London.
As part of a Covid vaccine injured group, Adam now works tirelessly to bring this suffering to the eyes and ears of media etc. He has already appeared on two American shows via zoom. He is now in negotiations with GB News, here in the UK. He is extremely active on Twitter – @oneadds – and has very many followers.
Adam is a changed man. He no longer yearns for the “life as it was ” but has, rather, accepted where he is at present and is working so hard for the good of all sufferers. His intention is to carry this on, in any way possible, to include MH issues too. We wish him well in all that he is doing.
Adam now finds that the Rugby League family are waking up to the present situation. He has a connection to them spanning many years, hence the article linked in above.
There is an important lesson here for us all, I feel. Recovery – in the sense of accepting the limitations put on us from unexpected directions and moving on – can reap rewards. We can’t all be in touch with Sports injury Clinics and get their support but, we can all offer hope in the darkest hours of suffering. Adam often says that, without the support given both in individual exchanges and in group sharing of concerns, he isn’t sure where he would be today. He turned to DH in his original hour of need. He was directed to our Withdrawal Group for support. We encouraged in a way that all human beings can and should do. All small steps in their own rights but, put together, they have enabled one man to flourish.
I think there is much yet to come in Adam’s story and we wish him the very best – for his own health and also for the health of all vulnerable beings.
Keep going Adam – we are all behind you!
Access to the bmj is free Rapid responses (comments) are also published alongside articles Anybody can make a rapid response
BMJ Investigation
FDA oversight of clinical trials is “grossly inadequate,” say experts
BMJ 2022; 379 doi: https://doi.org/10.1136/bmj.o2628 (Published 16 November 2022)
Cite this as: BMJ 2022;379:o2628
Responses
Maryanne Demasi, investigative journalist
Author affiliations
maryannedemasi@hotmail.com
Covid-19 vaccines and drugs were developed at “warp speed” and now experts are concerned that the US Food and Drug Administration inspected too few clinical trial sites. Maryanne Demasi reports
On 25 September 2020, the US Food and Drug Administration (FDA) received a complaint by Brook Jackson who had been working for Ventavia Research Group, a Texas based company hired to run clinical trials for Pfizer’s covid-19 mRNA vaccine. Jackson, a regional director, had witnessed problems at three trial sites she was overseeing and complained to an FDA inspector about a range of problems including falsified data, unblinded patients, and inadequately trained vaccinators who were slow to follow up on adverse events. “I thought that the FDA was going to swoop in and take care of everything. What I was reporting was so important,” Jackson told The BMJ. The FDA did not, however, inspect the trial sites in question.
This lack of oversight was not an isolated case, The BMJ has learnt. Regulatory documents show that only nine out of 153 Pfizer trial sites1 were subject to FDA inspection before licensing the mRNA vaccine. Similarly, only 10 out of 99 Moderna trial sites2 and five of 73 remdesivir trial sites3 were inspected.
Now, facing a backlog of site inspections, experts have criticised the FDA’s oversight of clinical trials, describing it as “grossly inadequate.” They say the problem, which predated covid-19, is not limited to a lack of inspections but also includes failing to notify the public or scientific journals when violations are identified—effectively keeping scientific misconduct from the medical establishment.
The FDA is “endangering public health” by not being candid about violations that are uncovered during clinical trial site inspections, says David Gortler, a pharmacist and pharmacologist who worked as an FDA medical reviewer between 2007 and 2011 and was then appointed as a senior adviser to the FDA commissioner in 2019-21.
“The lack of full transparency and data sharing does not allow physicians and other medical scientists to confirm the data independently and make comprehensive risk-benefit assessments,” continues Gortler, who is now a fellow at the Ethics and Public Policy Center thinktank in Washington DC.
Paused during the pandemic
Between March and July 2020, at the peak of pandemic restrictions, the FDA paused its site inspections and only “mission critical” inspections were carried out. Gortler says, however, that this was the time that the FDA should have ramped up its oversight, not scaled back, especially since covid-19 products were being developed at warp speed and intended for millions of people. “The drug companies took appropriate measures to keep staff safe, which is exactly what the FDA could and should have done,” said Gortler.
A former staffer in the FDA’s Office of Criminal Investigations was also concerned about the agency’s failure to fully tackle Jackson’s complaint about falsified data in Pfizer’s mRNA vaccine trial. In an email dated March 2021, they wrote, “Having worked at the FDA, I see it as surprising, for many reasons, that the agency turned a blind eye . . . They likely feared the criticism they undoubtedly would have received for holding up a vaccine (which they knew they would eventually approve anyway) at the expense of untold lives lost.”
The former FDA employee, who signed a non-disclosure agreement and did not respond to interview requests, went on to write, “My point here is that instead of the regulators protecting the public, they were complicit. At the time, they may have been doing what they believed to be the right thing under extraordinary circumstances. But now, they may soon have some explaining to do.”
The FDA told The BMJ it takes oversight of clinical trials seriously and had adapted to travel restrictions, publishing draft guidance4 for remote regulatory assessments. This guidance describes virtual inspections using live streaming and video conferencing and requests to view records remotely.
Gortler, who is a credentialed FDA inspector, laughed at the proposition. “You can’t do a remote inspection. That’s like saying I’m going to arrest somebody remotely. You have to be there on site and look at every nuance such as cleanliness, organisation, staff coordination—even their body language. During a pandemic, the FDA could’ve put inspectors in hazmat suits if they wanted to, there’s no excuse for not going onsite.”
Historical failure to oversee
The FDA has a long history of failing adequately to oversee clinical trial sites. A report in 2007 by the Department of Health and Human Services’ Office of the Inspector General found the FDA audited less than 1% of the nation’s clinical trial sites between 2000 and 20055 and was highly critical of the agency because it did not have a database of operational clinical trial sites.6 In response to the report, the FDA said it created a dedicated task force and “developed new regulations and guidance further to improve the conduct of clinical trials and enhance the protection of people participating in clinical trials.” The BMJ asked to interview a member of this task force, but the FDA denied our request.
In 2015, Charles Seife, professor of journalism at New York University, conducted an analysis of published clinical trials between 1998 and 2013 in which an FDA inspection found significant evidence of objectionable practices.7 A total of 57 published clinical trials had significant evidence of one or more problems: 39% had falsification or submission of false information, 25% had problems with adverse events reporting, 74% had protocol violations, 61% had inadequate or inaccurate recordkeeping, and 53% failed to protect the safety of patients or had problems with oversight or informed consent. Furthermore, only 4% of the trials that were found to have significant violations were mentioned in the study’s journal publications.
A 2020 Science investigation analysed the FDA’s enforcement of clinical research regulations between 2008 and 2019 and concluded the agency was often light handed, slow moving, and secretive. It said that the FDA rarely levelled sanctions and when it did formally warn researchers about breaking the law, it often neglected to ensure that the problems were remedied.8
It has been 15 years since the Office of Inspector General report and the FDA still has no record of how many clinical trial sites are operating across the US and abroad. The agency told The BMJ it does not compile an annual list of clinical investigational sites submitted for review because it is “resource intensive.”
“It’s unacceptable,” said Gortler. “All it would take is sending a blanket communication to all sponsors or applicants requesting they provide a list of all their international and domestic clinical trial sites. Also, the FDA should publish the names, inspection dates, and unredacted findings at each of these sites clearly on its website, not buried somewhere, nearly impossible to find.” He believes the agency should have implemented a policy decades ago. “The public has a right to learn immediately about any violations before choosing to use an FDA regulated product,” he says.
Some 65% of the FDA’s funding for the evaluation of drugs comes from industry user fees and in return the agency has mandated deadlines for decisions on new product applications. Some experts argue this has been a major contributor to the FDA being rushed and having insufficient resources for other critical activities.9
Insufficient staff and low morale
Historically, the FDA has faced challenges recruiting and retaining sufficient medical staff to meet its needs. According to a Government Accountability Office report published in January 2022, the turnover rate for FDA staff in key scientific areas was twice that of other government agencies in 2007, leaving the agency unable to fulfil its mission.10 Around 70% of the FDA’s career employees working in 2008 were eligible to retire by the end of 2014. Moreover, in 2018, medical product staff in “mission critical” occupations had salaries that were at least 20% lower than the average private sector salary for the same occupations, contributing to low morale. A news report said the pressures of work during the pandemic had led to two FDA reviewers dying by suicide.11
Despite the estimated hundreds of thousands of clinical trial sites in operation across the US and abroad, the FDA told The BMJ that it only has 89 inspectors for its bioresearch monitoring programme, which assure the quality and integrity of data submitted to the agency in support of new product approvals and marketing applications. The FDA told The BMJ it is recruiting more inspectors to reach its yearly average of 100.
“I don’t think that it is a sufficient number of staff to do that kind of level of oversight,” says Jill Fisher, professor of social medicine at the University of North Carolina. “The FDA must have enough of a presence to dissuade investigative sites from committing fraud,” she continues.
Secrecy of inspection findings
Occasionally, the FDA will uncover objectionable practices, such as failure to obtain informed consent, falsification of data, or violations in adverse event reporting.
The FDA publishes its inspection reports12 but the database is not comprehensive, nor are the reports proactively disclosed. When they are disclosed there can be extensive redactions making it difficult to link problems to a particular drug or clinical trial. “FDA redactions can render the document useless—it’s to the point of being comical,” says Gortler, whose current work focuses on FDA oversight and accountability. “Public health information should not be redacted like that,” he says.
The FDA does not typically notify journals when a site participating in a published clinical trial receives a serious warning, or alert the public about the research misconduct it finds.13
Rafael Dal-Ré, physician epidemiologist at the Health Research Institute-Fundación Jiménez Díaz University Hospital, Madrid, Spain, finds this concerning14 and points to the example of the anticoagulant drug rivaroxaban.
The FDA inspected trial sites of the Record 4 trial and identified serious deficiencies at eight of the study’s 16 trial sites.14 The violations were so numerous and severe that the FDA excluded the trial from its pile of evidence during the drug’s approval. But when the study was published in the Lancet in 200915 there was no mention of the data integrity problems and the paper has been cited more than 1100 times by others.14 When The BMJ sought comment from the authors of the Record 4 study, some said they were not fully aware of the data integrity problems prior to our inquiries. The lead author, Alexander Turpin, said he was seeking more information from the drug company. The Lancet told The BMJ it was looking into the matter.
“If research misconduct is identified, the FDA may reject the affected data from the product’s safety and efficacy evaluations, but then fail to disclose these data in the product labelling,” added Dal-Ré.
Gortler finds it unconscionable that the FDA withholds this information from the public. “Misconduct should be released immediately. It’s malpractice not to; it’s irresponsible,” he says.
In response to the criticism the FDA said that it does not always monitor all publications of data that were submitted to the agency, nor does it have the authority to demand that a journal retract an article.
Road to reform
Fisher says the FDA needs better resourcing. “The clinical trials industry has become a complex global enterprise, and the FDA does not have the resources to oversee all the research that is happening, even within the US. The FDA needs to be better funded and staffed to conduct inspections. At a minimum the agency needs to inspect sites when complaints or concerns have been filed,” she says.
Gortler doesn’t agree, however, that the FDA is under-resourced. With a total budget of $6.1bn in 2021, he suggests the agency needs to be leaner and more efficient, with employees interested in improving public health. “The bottom line is that the FDA has over 18 000 full time employees, more than any other drug regulatory agency by far, so it could have retrained and retooled anybody to tackle the need for increased inspections,” he says. “Half of its budget, about $3bn, is discretionary, which means it could have hired contractors, retirees, or repurpose existing workers. It chose not to. The FDA was just yawning its way through the pandemic. The entire agency is broken.”
“It felt like we were being told to hide things”: FDA’s approval of antibiotic Ketek
In 2004, the FDA approved Sanofi-Aventis’s new antibiotic Ketek (telithromycin) for outpatient treatment of community acquired respiratory tract infections. Since then, it has been implicated in hundreds of reported cases of severe liver injury and dozens of deaths, triggered two Congressional hearings, and led to reforms of the agency’s processes. In 2007, the FDA added a warning to Ketek’s label and removed all indications except for bacterial pneumonia.
David Ross was an FDA medical reviewer who led the initial safety review for Ketek in 2001, as part of a 10 year career at the agency’s Center for Drug Evaluation and Research. In his original review Ross, now an associate clinical professor of medicine at George Washington University School of Medicine and Health Sciences, found that Ketek’s risks included liver injury and other serious adverse events that were concerning given the millions of antibiotic prescriptions written annually for respiratory tract infections.16
In 2001, the FDA recommended to Sanofi-Aventis that the company gather additional safety data. Sanofi-Aventis conducted Study 3014, a 24 000 patient safety study done in only five months. The FDA’s limited resources only allowed one out of 1800 sites to be inspected initially.
The agency decided to inspect the highest enrolling site, reasoning that failure to find any problems there would allow all the other sites to be considered clean. “The FDA inspector found evidence of blatant fraud almost immediately. For example, patients being enrolled at times when the clinic was supposedly closed,” said Ross.
The inspector reported her findings to FDA’s Office of Criminal Investigations, with serious protocol violations subsequently found at several other high enrolling sites. Eventually, the site investigator pleaded guilty to fraud and served a 57 month prison sentence.
At a 2003 public meeting of the FDA’s anti-infective advisory committee, data from Study 3014 were presented to the panel without disclosing the numerous violations and data integrity problems found at the initial trial site, which sparked a criminal investigation. Janice Soreth, Ross’s division supervisor at the time, has previously said that there was no intention to deceive the committee and that the violations were not disclosed so as not to compromise the ongoing criminal investigation.17 But Ross says he was appalled: “I felt like we were being told to hide things from the advisory committee.”
Unaware of the integrity problems, the committee voted 11 to 1 to recommend approval of Ketek. The FDA granted the drug approval on 1 April 2004. In a memorandum from the FDA, the agency said it was “difficult” to rely on Study 3014 for its approval because of the data integrity problems, instead using spontaneous adverse events reports for its understanding of Ketek’s overall risk-benefit profile, which goes against standard drug review practice.18 The first Ketek associated death from liver injury was reported to the FDA seven months later.16
A series of events unfolded during the drug approval process, which would later be revealed at a Congressional hearing in 2007. Ross testified under oath that when he submitted his follow up safety review in 2004, concluding that Ketek carried far too much risk to ever be approved for relatively minor conditions such as bronchitis and sinusitis, Soreth asked him to “soften” the language so that it would give the leadership “more wiggle room.” He told the hearing he sent Soreth a revised version for signing but—without telling her—also put the original in the electronic archive. Soreth did not testify in the hearing and denies this allegation. “No one ordered a change in Dr Ross’s review. He was free to keep his original draft,” she told The BMJ. “His review, moreover, did not include Aventis’s final submission to the agency.”
Ross left the division after Ketek’s approval in 2004 and then left the FDA in 2006, saying “the FDA did nothing for months and they just watched as the adverse event reports piled up. Lives could and would have been saved if the FDA acted sooner than it did to publicise Ketek’s risks and put a boxed warning on the drug.”
Whether it was Ross or Soreth who was right about this, the Ketek controversy led to the FDA Amendments Act of 2007, which stated that a reviewer’s work “shall not be altered by management or the reviewer once final.”
The FDA declined to respond when contacted by The BMJ. A spokesperson for Sanofi said the company complied with all the investigations at the time and that it no longer sells Ketek.
BMJ Investigation
FDA oversight of clinical trials is “grossly inadequate,” say experts
BMJ 2022; 379 doi: https://doi.org/10.1136/bmj.o2628 (Published 16 November 2022)
Cite this as: BMJ 2022;379:o2628
All rapid responses A very rapid response from Peter Selley retired GP. It’s always worth reading his responses
Dear Editor
Maryanne Demasi describes the low-key approach to the FDA’s supervision of covid-19 vaccine trials.
The Pfizer trial had in fact 130 research sites in the USA, but still less than 7% of these sites were inspected by the FDA (1).
Worldwide there were 153 sites. Pfizer conducted their own audits (2), but only in 23 sites (15%).
Seven of these audits were described as “non-routine”. These included the sites in Fort Worth (five weeks after Brook Jackson’s complaint to the FDA (3)) and in Argentina, which contributed 13% of the total trial participants and was the only centre to be audited twice by Pfizer.
The requirements for an internal non-routine audit are not specified.
The trial sponsors should have a duty to disclose the outcomes of these audits.
1 of 1
Censorships and their Antidotes in Covid Times
Inbox
Josh Guetzkow from Jackanapes Junction
1:16 PM (25 minutes ago)
Josh Guetzkow
Nov 17 · Jackanapes Junction
Two titans of evidence-based medicine chime in on our paper on censorship and suppression of COVID-19 heterodoxy.
Censorships and their Antidotes in Covid Times
Persecution of those with differing views is alive, well and kicking. Energised by the Covid pandemic.
TOM JEFFERSON AND CARL HENEGHAN
NOV 17
….., the persecution principle is alive and kicking. It has been energised but also brought to the surface by the Covid pandemic.
A recent publication on censorship and suppression and its tactics and counter-tactics drew our attention. The study was based on interviews with established scientists “who were censored for their heterodox views on COVID-19”.
Participants reported 12 censorship and suppression tactics used by the medical establishment and the media due to their critical or unorthodox positions on COVID-19. Our analysis is that these fall into three broad categories: Silencing and Censorship, Denigration and Discrediting of an individual and Complaints and Intimidation.
There are also other tactics coming to the fore that are context specific. Shadowbanning occurs when social media platform stops a user’s content from showing up without notifying the user.
The reactions of the victims were interesting: intimidation did not seem to work, all those interviewed carried on articulating their work and thoughts, some took legal actions, and others formed support networks. Perhaps the most interesting reaction was the use of alternative means of communication. Coming under attack requires highly effective communication strategies to get your points across.
Silencing and Censorship can be addressed by finding alternative sources of communication (e.g substack) and media outlets that are better at dealing with and expressing uncertainties. Denigration and Discrediting require keeping calm and not responding in kind while sticking to an evidence-based approach – counter certainty with uncertainty. Complaints and Intimidation are perhaps the most pervasive strategies and most difficult to deal with. But take advice, confide in trusted sources and play the long game.
…… But lockdown sceptics were vilified by people such as the Tory MP Neil O’Brien, with his Covid-19 FAQ website behaving as if it was the authority on all things pandemic.
This was a website which sought to humiliate scientists such as Sunetra Gupta, Carl Heneghan and Tom Jefferson for daring to challenge the Sage groupthink. In general, journalists who questioned the wisdom of shutting down classrooms were treated like pariahs, accused of putting teachers’ lives at risk. Yet according to the Office for National Statistics, there were just 139 Covid deaths in teaching and educational professionals aged 20 to 64 years from March to December 2020 in England and Wales. Even after schools reopened, coronavirus-related deaths for this group were “statistically significantly lower” than the average.’
Academic and Journalistic freedom requires individuals to pursue knowledge wherever it may lead without undue or unreasonable interference. The scientists and doctors in this present study did not refrain from expressing their points of view that others considered objectionable. The ability to overcome such self-censorship is fundamental to a democracy and its capacity to make decisions in the best interest of its citizens.
Trust the Evidence is a reader-supported publication. To receive new posts and support our work, consider becoming a free or paid subscriber.
Nuremberg II: What a Real Inquiry into the Response to Covid Would Look Like
Disturbing questions remain as to who did what, when, and why in the days leading up to the lockdowns of spring 2020 and beyond.
Michael P Senger
Oct 11
In the aftermath of the world’s catastrophic response to Covid-19, some governments have begun conducting inquiries into what went wrong. Yet owing to a combination of politics, face-saving, and outright corruption, these inquiries have generally been toothless. For example, a report published last year by the UK House of Commons concluded, backwardly, that if the UK had gone into strict lockdown three days sooner, disaster would have been averted.
Conclusions like these are as insulting to the public’s intelligence as was the response to Covid itself. The response to Covid led to the sharpest economic collapse since the Great Depression, global famine, a mental health crisis, runaway inflation, a transfer of over $3 trillion from the world’s poorest to the very richest, the premature deaths of hundreds of thousands of young people, and the worst education crisis since the Second World War.
Given the magnitude of the harm that’s been done, the public deserves to know exactly who knew and did what, when, and why in the days leading up to the lockdowns of spring 2020 and beyond. Though it may not be politically feasible, ideally this would one day take the form of an international tribunal. Below are just some of the many disturbing questions to which any leader who claims to represent the public ought to demand answers:
Why did the CDC suddenly adopt “measures to increase social distance” as official policy in 2004, contrary to all the epidemiological guidance it had developed throughout the 20th century?
Who was behind the campaign to export the concept of “lockdown” to Liberia and Sierra Leone in 2014?
Some intelligence reports have indicated that members of the western national security community were aware a new virus had emerged in China by fall 2019. What was being said about the virus at that time?
If some national security officials had been worrying about a new virus in China since fall 2019, how could they have possibly believed China’s two-month lockdown of Wuhan eliminated the virus several months later?
By January 2020, tips began to emerge that the World Health Organization was planning to recreate China’s lockdowns across the world, starting in Italy. When and on what basis did the World Health Organization make this decision?
Lockdowns had been ruled out by the pandemic plans of the WHO and every developed nation. Why weren’t these pandemic plans followed?
Why were health security officials talking about “curfews of indefinite duration” by February 24, 2020?
Why does the WHO’s February 2020 report rely on logical fallacies in its promulgation of China’s lockdown measures as global policy?
Why was the current Director of National Intelligence sitting next to China’s CDC director at the Event 201 simulation of a coronavirus pandemic in October 2019, shortly before a real coronavirus pandemic emerged?
Former White House Coronavirus Response Coordinator Deborah Birx has made conflicting statements about how she got her job. For example, the former Deputy National Security Advisor offered her a job in the White House as public health security advisor as far back as November 2019. How was Birx chosen for this role?
Who was behind the terror campaign of fake videos showing Wuhan residents spontaneously dying and convulsing in the streets in January and February 2020?
Why is there no record of the hero doctor Li Wenliang before he appeared in Chinese state media at the end of January 2020? On what basis did western media outlets adopt this story as true?
High-level members of the national security community including the former Director of National Intelligence and the former Secretary of State have stated as fact that Covid came from a lab in Wuhan. At the same time, high-level scientific officials including NIAID Director Anthony Fauci have stated that it is “molecularly impossible” for Covid to have come from that lab. How can we still have this disconnect at the highest levels of the federal government?
A report revealed that military leaders saw Covid as a unique opportunity to test propaganda techniques on the public. Who advised western leaders to use military-grade propaganda on their own people?
Some officials in the UK later expressed contrition about the fear campaigns that the UK Government used on its own people to convince them to support Covid mandates. How was the decision to use these fear campaigns made?
Who was behind the massive bot and astroturf campaigns to popularize lockdowns among western citizens and officials in March 2020?
What was the origin of international slogans such as “follow the science,” “together apart,” “just stay home,” and “two weeks to slow the spread” which were used to drive support for Covid mandates?
How many people were killed by the WHO’s initial guidance on mechanical ventilators based on Chinese journal articles advising ventilators as the “first choice” for those hospitalized with Covid?
The initial guidance from the WHO advised using mechanical ventilators not necessarily for the patient’s benefit, but to control the spread of the virus. Why was the WHO advising doctors to violate the Hippocratic Oath?
Why were numerous, credible predictions of famine, human rights disasters, and economic collapse as a result of lockdowns ignored?
Why was natural immunity so long ignored?
Why were initial seroprevalence studies downplayed?
Why were beaches and other outdoor spaces closed?
Why was the public kept in the dark about low early estimates of Covid’s actual infection fatality rate?
What was the source of the guidance to move patients who were still sick into nursing homes?
Remdesivir and midazolam were initially widely used, but didn’t lead to positive health outcomes. How was the decision made to use these over other treatment protocols?
Leading officials have made conflicting statements as to whether the goal of lockdowns was to eliminate the virus, slow the spread, or buy time for vaccines. What was the actual goal they had in mind at the time they implemented these policies?
Why did key public health officials make statements about using the response to Covid to advance non-health-related policy goals?
How was the decision made to suppress and censor scientific opinions that dissented from lockdowns?
Why were so many federal officials so intimately involved in the censorship of dissenting opinions on social media?
Why did elite western newspapers, media networks, and public health leaders so diligently repeat the absurd line that China had eliminated Covid by shutting down one city for two months?
Why did elite western publications begin explicitly urging the public to adopt a response to Covid that was more like China’s?
Why were mechanical drones initially deployed by various states and countries to monitor lockdown compliance?
What accounted for the close international synchronization of Covid mandates?
Why did masks shift from being not advised to being mandatory?
The New York Times confirmed that at the standard cycle threshold level used for PCR testing, 85 to 90% of Covid cases were false positives. How did this practice become standard?
Why were widely-known and publicized problems with PCR testing and comorbidities ignored for purposes of counting Covid deaths?
Why did key public health officials so quickly shift from saying vaccines would prevent Covid to saying proof of vaccination should be mandatory to partake in everyday activities?
Why has there been so little public discussion of China’s influence on the global response to Covid, despite FBI Director Christopher Wray’s disclosure that Chinese officials were “aggressively urging support for China’s handling of the COVID-19 crisis?”
Why was the UK Government so deferential to Neil Ferguson and Imperial College during the response to Covid despite Imperial’s close relationship with China?
Why has the editor-in-chief of the Lancet been so publicly deferential to China?
Why did Bill Gates express such admiration for China’s response to Covid?
Why did the German government privately disseminate a list of authoritarian measures provided in part by China lobbyists?
How did a 40-year member of the British Communist Party with no background in epidemiology become a leading advisor to the UK Government, and why was she recently promoted to lead the WHO’s nudge unit?
Why did leading economists assume that a short, sharp lockdown would “eliminate the resurgence risk” when the policy had no precedent?
Why did the Federal Reserve and its international counterparts disregard inflation?
Why did the Supreme Court and its international counterparts step aside while lockdowns were being implemented?
Why did the judiciary acquiesce to an indefinite state of legal emergency?
Why did western politicians and public health officials demonstrate so little concern for following their own Covid rules?
If the virus was deadly enough to kill millions and justify an indefinite state of emergency, why has so little effort been expended to hold China accountable for its initial coverup of that virus?
Though many in positions of power would prefer that we forgot, the strict lockdowns that consumed the world in 2020 are extremely well documented. Above all, these lockdowns were a chilling demonstration of just how quickly western officials, policymakers, scientists, journalists, and soon entire populations could be convinced to adopt a degree of totalitarianism in their everyday lives. Until we have real answers as to how exactly they happened, and why, there’s no reason for any thinking citizen to have confidence in the current crop of officials who claim to represent them.
Michael P Senger is an attorney and author of Snake Oil: How Xi Jinping Shut Down the World. Want to support my work? Get the book. Already got the book? Leave a quick review.
The New Normal is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid sub
Dr Clare Craig (not one of her impersonators)
@ClareCraigPath
·
1h
MHRA still trotting out the ludicrous 20 million lives saved.
https://www.gov.uk/government/news/second-pfizerbiontech-bivalent-covid-19-booster-vaccine-approved-by-uk-medicines-regulator
It’s fiction.
https://www.hartgroup.org/imperial-fantasy-of-20-million-lives-saved/
They are living in Cloud-Covid-Land. The public need to realise that institutions such as Imperial receive considerable funding from the pharmaceutical industry and this colours how evidence is presented. The mainstream media are claiming such evidence is “science” when it is not much more than marketing for the pharmaceutical industry. The chasm between reality and the mainstream narrative is widening and the public need to realise that sources they have trusted in the past cannot be trusted on this topic any longer.
Dr Clare Craig (not one of her impersonators)
@ClareCraigPath
·
1h
In this MHRA table, facial paralysis is included as more common than myocarditis as an adverse effect of Pfizer.
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1116355/SPC_COMIRNATY_bivalent_original_omicron_BA1_clean.pdf
Table: P.7 of 21
Dr Clare Craig (not one of her impersonators)
@ClareCraigPath
·
1h
They based their assessment of how common on the four cases in the trial! Have they done no work on this in the last two years of rollout to find an actual rate?
Dr Clare Craig (not one of her impersonators) Retweeted
Bernie’s Tweets
@BernieSpofforth
BILL GATES – “People act like they have a choice” He wasn’t joking he was warning you. The declaration by the G20 will attempt to ensure you all comply! With whatever they determine.
https://twitter.com/BernieSpofforth/status/1593188470692139010
“when we’ve largely vaccinated the entire global population” – Bill Gates
Why Has the Government Never Apologised to Those Who Took the Vaccine ‘To Protect Others’ via a Herd Immunity That Never Came?
BY AMANUENSIS
17 NOVEMBER 2022 7:00 AM
https://dailysceptic.org/2022/11/17/why-has-the-government-never-apologised-to-those-who-took-the-vaccine-to-protect-others-via-a-herd-immunity-that-never-came/
‘Of course, these rigorous studies are conspicuous by their absence, with mainly lower quality ‘observational’ studies having been undertaken. It is surprising that so many senior scientists in the U.K. and worldwide have remained quiet about the lack of rigour of Government sponsored studies into the impact of the vaccines. These individuals have also remained quiet about the value in striving to achieve very high vaccination rates if the vaccines don’t offer protection from infection (no herd immunity possible) and at best only offer protection from hospitalisation or death (both very low risks for the non-vulnerable population).
Alas, it turned out that the vaccines haven’t offered meaningful protection from infection, and thus onward transmission, and ‘herd immunity’ is a phrase that appears to have entered the memory-hole. The appeals to ‘get vaccinated’ slowly changed from ‘protect yourself’ to ‘protect granny’ and then to ‘protect the NHS’, without any real explanation to the public as to why these messages had to change. I have given up thinking that it is odd that our authorities haven’t apologised to all those who took the experimental vaccines only ‘to protect others’ – indeed, the situation is actually that the vaccines were rolled out to achieve herd immunity and it is only by a fluke that they’ve turned out to offer some protection against hospitalisation and death (if indeed this is the case; there’s even evidence that the vaccines are making hospitalisation and death from Covid more likely). Our authorities are, of course, very keen to tell everyone that the vaccines were always intended to achieve this end result, even with very little evidence from the original vaccine trials that this was occurring.
I sometimes wonder why anyone under the age of 60 or so is getting vaccinated at the moment.’ …
Albert Bourla
@AlbertBourla
·
Thank you @matthewherper for an invigorating discussion at #STATSummit…..
https://twitter.com/AlbertBourla
Matthew Herper
@matthewherper
https://twitter.com/matthewherper/status/1593430580582125571
Matthew Herper Retweeted
Albert Bourla
@AlbertBourla
·
Thank you @matthewherper for…..
Meteroric, Innovate and Protect…
CRUCIAL QUOTE
“The Covid-19 vaccines, including boosters, continue to save countless lives and prevent the most serious outcomes—hospitalization and death—of Covid-19,” FDA Commissioner Robert Califf said in the FDA’s approval of the bivalent vaccine, according to a release.”
The proposal may also face resistance from the drug industry after its meteoric race to develop vaccines and treatments, which were critical tools in controlling the virus which has killed more than 6.5 million people worldwide.
Pfizer and its partner BioNTech, Moderna and AstraZeneca tested, developed and launched vaccines less than a year after the virus first emerged in China in December 2019.
Thomas Cueni, Director General, International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), said the draft was an “important milestone”, but added that it was important not to undermine how pharmaceutical companies innovate and to protect their intellectual property (IP).
https://www.msn.com/en-gb/news/world/big-pharma-may-have-to-reveal-government-deals-in-who-s-draft-pandemic-rules/ar-AA14et3s?ocid=msedgdhp&pc=U531&cvid=cb195663a0b145bdb9d13e8bf0e5db72
New Pfizer-BioNTech Covid Vaccine Is More Effective Than The Original, Study Shows
https://www.forbes.com/sites/tylerroush/2022/11/18/new-pfizer-biontech-covid-vaccine-is-more-effective-than-the-original-study-shows/?sh=3079b1b37c24
TOPLINE
Pfizer-BioNTech’s new bivalent Covid-19 vaccine—which targets the original strain and some Omicron subvariants—provides more protection against the virus and its omicron variants than its initial vaccine, according to a study released Friday, as over 35 million people in the U.S. have since received the updated booster.
A booster dose of the Omicron BA.4- and BA.5-adapted Covid-19 vaccine was 11 times more effective against the BA.4.6 subvariant, nearly seven times more effective against the BA.2.75.2 subvariant, nine times as effective against the BQ.1.1 subvariant and almost five times more effective against the XBB.1 subvariant than the original vaccine.
The results complemented earlier data from Pfizer suggesting the new shot is 13 times more effective against the BA.4 and BA.5 subvariants—the most common versions of the omicron variant—in people over the age of 55.
The new booster was recently approved by the FDA for emergency use in children aged 5 or older.
According to the Centers for Disease Control and Prevention, over 35 million people aged 5 and up—or 11.3% of the U.S. population—have received an updated bivalent booster dose.
CRUCIAL QUOTE
“The Covid-19 vaccines, including boosters, continue to save countless lives and prevent the most serious outcomes—hospitalization and death—of Covid-19,” FDA Commissioner Robert Califf said in the FDA’s approval of the bivalent vaccine, according to a release.
TANGENT
Weekly cases of Covid-19 continue to decrease, according to the CDC, which reported a seven-day average of 39,000 new cases as of Nov. 2. The rate at which vaccines have been administered has also declined—despite 68.5% of the U.S. receiving a full vaccination—as 49.1% of booster-eligible people have yet to receive one.
KEY BACKGROUND
Both the Pfizer-BioNTech and Moderna bivalent Covid-19 vaccines have been authorized for use as a single booster dose in people over the ages of 5 and 6, respectively. The bivalent vaccine, which is referred to by the FDA as an “updated” vaccine, is a combination of an original virus strain with a component of an omicron variant to combat all possible versions of the virus. Pfizer has noted some possible symptoms as a result of the bivalent vaccine, including possible joint pain, fatigue and other allergic reactions.
Matthew Herper Former Staff
https://www.forbesindia.com/article/cross-border/glaxosmithkline-takes-its-medicines-how-andrew-witty-patched-up-the-sick-drugmaker/44413/1
Andrew Witty inherited a drugmaker sick with scandal and spent the next eight years patching up his patient. GlaxoSmithKline may finally be well again
“have a chicken dinner “ – Andrew Witty
Matt’s galore…
How does such a disreputable shifty bunch get to rule the world?!
Fom -The Sociable
HomeBusinessG20 should adopt WHO-standardized vaccine passports, digital identity schemes: B20 Summit
BUSINESSGOVERNMENT AND POLICYTECHNOLOGY
Digital ID, vaccine passports can give govts & corporations power to incentivize, coerce, or otherwise manipulate human behavior: perspective
Tim HinchliffeTim Hinchliffe7 days ago4 (He is not an antivaxxer)Commentsdigital idDigital Identityg20globalismperspectivesocial creditvaccine passports
Indonesian Health Minister Budi Gunadi Sadikin at B20 Summit 2022
The B20 calls on the G20 to adopt vaccine passports using WHO standards and to promote digital identity schemes.
Ahead of the Group of Twenty (G20) Summit on November 15, the Business 20 (B20) held its own summit in Bali, Indonesia from November 13-14.
On the first day of the B20 Summit, Indonesia’s Minister of Health Budi Gunadi Sadikin said that G20 countries should adopt a “digital health certificate using WHO standards” and that they were looking to introduce this type of vaccine passport into the “international health regulations” during the next World Health Assembly in Geneva.
“Let’s have a digital health certificate acknowledged by WHO — if you have been vaccinated or tested properly — then you can move around” — Budi Gunadi Sadikin, Indonesian Health Minister, B20 Summit 2022
“G20 countries have agreed this digital certificate using WHO standard, and we will sub it into the next World Health Assembly in Geneva as the revision to international health regulation” — Budi Gunadi Sadikin, Indonesian Health Minister, B20 Summit 2022
“Let’s have a digital health certificate acknowledged by WHO — if you have been vaccinated or tested properly — then you can move around,” said the Indonesian health minister.
“Indonesia has achieved, G20 country has agreed, this digital certificate using WHO standard, and we will sub it into the next World Health Assembly in Geneva as the revision to international health regulation.
“Hopefully for the next pandemic, we can still see some movement of the people, some movement of the goods, and movement of the economy,” Sadikin concluded at the B20 Summit.
Prior to becoming Indonesia’s Minister of Health, Sadikin worked in high-level positions at Bank Bali, Bank Mandiri, ABN AMRO Bank Indonesia, PT Bank Danamon, and IBM.
“Adopt the Digital Documentation of COVID-19 Certificates” — B20 Policy Recommendation, 2022
Those policy recommendations include, but are not limited to:
Adopting the Digital Documentation of COVID-19 Certificates
Creating robust guidelines on health emergency preparedness to ensure global coordinated response for future crises enhanced by a technology-enabled “always-on” global health infrastructure
In August, 2021, the World Health Organization (WHO) published a 99-page guide book on the implementation of digital documentation of COVID-19 certificates, aka vaccine passports, stating that “a health pass based solely on individual vaccination status may increase the risk of disease spread.”
This is because the COVID-19 “vaccines” were never proven to prevent transmission nor infection, and it recently came to light in the European Parliament that Pfizer never even tested its product for stopping transmission.
Despite this knowledge being publicly available, the B20 is still recommending proof of vaccination as a means to travel.
“These [vaccine] passports by nature serve as a form of digital identity” — World Economic Forum, February, 2022
Vaccine passports, by their very nature, serve as a form of digital identity, according to the World Economic Forum (WEF).
A digital identity encompasses everything that makes you unique in the digital realm, and it is a system that can consolidate all of your most personal intimate data, including which websites you visit, your online purchases, health records, financial accounts, and who you’re friends with on social media.
Now, the B20 Final Communique is also recommending, as a matter of policy, that the G20 support and promote not just vaccine passports, but overall public and private digital identity schemes as well.
These digital identity schemes are said to help bolster financial inclusion, mitigate crises, and serve as “a building block for data privacy and digital trust.”
However, digital identity schemes can give governments and corporations the power to incentivize, coerce, or otherwise manipulate human behavior under a system of social credit.
Source: World Economic Forum
“The G20 should ask MDBs [Multilateral Development Banks] to support governments in developing and implementing digital identity schemes to bolster financial inclusion” — B20 Policy Recommendation, 2022
These policy recommendations say that:
The G20 should adopt action plans to realize the benefits of inclusive digital payments systems through several actions such as digitizing government payments and actively engage in the regulatory agenda
The G20 should promote usage of the cloud within public and private entities
investments and public-private partnership on digital initiatives to detect fraud
The B20 is the official G20 dialogue forum with the global business community, and it is tasked with formulating policy recommendations on designated issues.
The recommendations are then delivered to the G20 Presidency at the B20 Summit, which takes place around the G20 Summit.
Source: Council on Foreign Relations (CFR)
The G20 is a forum comprising nineteen countries with some of the world’s largest economies, as well as the European Union (EU).
The countries are Argentina, Australia, Brazil, Canada, China, France, Germany, India, Indonesia, Italy, Japan, Mexico, Russia, Saudi Arabia, South Africa, South Korea, Turkey, the United Kingdom (UK), and the United States.
Spain is invited as a permanent guest, according to the Council on Foreign Relations.