
This is the second of a 3 lecture series given to family doctors in Stockholm on September 9 this year. The meeting was organized by André Marx. The alternate title of this talk is Girl who Tackles Ghosts. It follows Girl with a Clinical Trial Tattoo. It will be followed by Girl who Eats Salt.
Thomas Hultgren did a video recording through Zoom. One child makes a welcome appearance. It is HERE.
We have a lady in our waiting room. We still have to work out what might helping her mean.
Slide 36

Do you think studying monolayers of cells will tell you
why you fall in love with a girl?
Walter Hess was a Swiss neuroscientist involved in mapping the brain, who won the Nobel Prize for Medicine in 1949. The kind of person who trained people like Arvid Carlsson the Swedish inventor of the SSRIs. Fridolin Sulser, another big psychopharmacology figure, was one of Hess’ pupils and recalls him making comments about nerve cells and falling in love when training them. Hess’s point is that physiology is one thing, but the goal is to understand function – why we behave the way we do.
The quote also tells you the students then were all male. The drug world we now have is a male creation – perhaps not surprising that it focusses on efficacy rather than safety. Evidence Based Medicine is all about effective doing. A Relationship Based Medicine might be more about safety.
Slide 37
We need to update Hess to make clear that just as with neuroscience so with RCTs – the goal with any science is to understand why we engage in the relationships we engage in.
Perhaps with
Do you think studying RCT ‘data’ will tell you
whether to enter a relationship with this person or not?
How will RCTs help us relate to this strange person in the waiting room?
Slide 38
In Girl with a Clinical Trial Tattoo, Tony Hill told us that RCTs may help us with one thing – therapeutic efficacy. With antibiotics efficacy was obvious – it meant saved lives, people back to work. You can deliver a health service for free if the things you do save lives or get people back to work.
We are told antidepressants and lots of other drugs work – but in antidepressant RCTs more lives are lost on treatment and more people disabled rather than re-enabled. What’s up?
Slide 39

Most RCTs have surrogate outcomes to indicate if a drug works – such as lipid or sugar blood levels. In mental health, the indicators are checklists – we call them rating scales – but Max Hamilton, whose name is on the famous Hamilton Depression Rating Scale, viewed his scale as a prompt to questions to include in an interview.
One advantage to industry of the HAM-D is that it rolls many of the adverse effects of treatment into features of the illness. To work out whether the suicidality or loss of libido on treatment is caused by the illness or the drug needs an act of judgement. If an adverse effect affects the score on the scale the HAM-D numbers become meaningless.
Besides that serious problem, Hamilton is raising another serious question here. He could see that using checklists risked standardizing clinical encounters. Checklists risk turning relationships into a commodity – as happens in Fast Food outlets, where the staff are programmed to say certain things to deliver quality relationships along with your quality hamburger.
Slide 40

For industry, a quality hamburger is one that is the same every time. Its not the best hamburger you have ever had or even a very good one. For Pharma and health services, quality relationships are the same every time.
Rating scales were for trials – not clinical practice. The same is true of lipid and sugar levels and Blood Pressure. What Hamilton foresaw shows up in lots of ways in clinical practice today. We have clinical encounters that look like the image in this slide.
Instead of relating, clinicians are forced by services to assess people in ways that are superficially scientific but end up selling products – rather than encounter people with a view to helping them lead the life they want to live.
Rating scales constrain us to get patients to live the lives Pfizer or GSK or a health service company wants them to live.
Slide 41
It shows up again in these two slides. Using checklists, we turn everything we now touch to objective scientific Gold.
The ancient Greeks foresaw the problem and that only someone stupid would wish for something like this.
Slide 42
Now I want to introduce you to another angle on all this. In terms of what you are about to hear, the best thing you can read is Malcharist. It’s a thriller. A Fiction. But it comes horribly close to being the truth behind the scenes of health services today.
The cover shows a medical Ghostwriter having a crisis of conscience. The greatest concentration of Fake literature on earth never centred on Donald Trump – it centres on the drugs you give your patients.
Slide 43
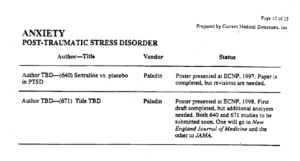
In the 1970s, Pharma outsourced the writing of articles reporting clinical trial results to medical writing – ghostwriting – companies. Running Clinical Trials was outsourced to Contract Research Organizations (CROs), now a $40 billion business. CROs are the ones who code and collate the figures collected in trials passing them on to the ghostwriters. The actual clinical trial paperwork – possibly files now – ends up in offshore or online data havens.
This slide is from a document produced by a company doing the ghostwriting of articles on Zoloft for Pfizer – see Cattell for more. You see on the right – 2 PTSD articles have been written and the company knows where these will go – to the very best journals in the field. None of these journals are bothered about whether the patients actually exist. None ask to see the data.
On the left you see TBD – To Be Determined. Who the authors will be needs input from Pfizer’s marketing department. The authors need not be people who ran the experiment. Neither these authors, nor the people who ran the study, nor the ghostwriters have access to either the people in the trial or the figures. The authors will be the people who Pfizer marketing think will do the best job selling Zoloft.
Slide 44

Just like Tony Hill was surprised to find drug companies promoting RCTs – so now drug companies strongly support Evidence Based Medicine (EBM). When EBM began in the early 1990s, people figured that this was a way to control pharma – just like Louis Lasagna thought RCTs were a way to control pharma.
Now doctors don’t have obvious pharma people in their office giving them free lunches anymore, or if they do these reps will encourage doctors to be evidence based. Pharma now have totally independent groups writing guidelines to do this job for them – like the National Institute for Healthcare and Clinical Excellence (NICE). And medical job contracts increasingly mandate that we adhere to these guidelines.
Where drugs are concerned, these guidelines are based on a ghostwritten literature. The people who write them have no access to the data from any of the trials they cite as evidence in support of what you or I are supposed to do. Guidelines only talk about Efficacy – Benefits – not Hazards.
I have written to the Chair and CEO of the NICE guidelines in the UK, regulators in the UK and Europe, politicians and others and none of them deny these points. Their response is one that I have given them – that it’s not their job to police the medical literature. See Crack of Doom.
All of the correspondence is available on the Politics of Care Forum on dhblog.wpengine.com
Slide 45
There are some key dates in the transition away from What Used to be Called Medicine to the health service jobs most of us have now. One date was September 20, 1991 – almost exactly 30 years ago. All through the year before, there were convincing reports of people given Prozac, becoming suicidal, with the problem clearing when Prozac was stopped, and reappearing when it was restarted. See Let Them Eat Prozac.
FDA were forced to have a hearing on antidepressants and suicide. Eli Lilly pitched their RCTs in response stating:
- the plural of anecdote is not data
- it’s the disease not the drug
- are you going to believe the anecdotes or the science?
On the day of the FDA meeting, the BMJ, the most pro EBM medical journal, published Lilly’s meta-analysis of its RCTs, which claimed to show no increased suicide risk.
The article shows an increased number of suicidal events on Prozac – but in the main paper this was not statistically significant which meant BMJ were happy with a statement that the problem didn’t exist. BMJ must not have read the small print in a footnote of the article they published which contained an admission about an ‘illegal’ coding of one event. Reverse this and the suicidal events were statistically significant.
FDA talked about heart-breaking cases reported to them but concluded the science didn’t support the drug causing the problem. FDA meanwhile were sitting on data for sertraline and paroxetine showing the same very limited efficacy compared with older antidepressants, like imipramine, and increased suicidal events rates on all three SSRIs with Lilly, Pfizer and SmithKline breaching FDA regulations to hide the problems.
FDA opted not to warn because warning would put people off against seeking the benefit – from a treatment that according to them had a favourable benefit-risk ratio.
From this point on the hazards of drugs began to vanish. Journals like the BMJ became scared to take case reports which previously had been the bedrock of medicine. Drugs and Therapeutics Bulletins vanish – soon replaced by Guidelines which only list benefits.
Doctors have now lost a sense for drug induced toxicity which is often coloured by anxiety or depressive symptoms. There are no treatments licensed for toxicity and so faced with someone saying they feel anxious or depressed as you can do when you have the Flu, are pregnant, or have a toxic reaction to drug, doctors increasingly give more of the drugs that are causing the problems in the case of psychotropic meds.
This is like giving someone with encephalitis an antidepressant or antipsychotic.
As a result Patients have become Invisible. We don’t hear or see them in the way we used to.
If patients become invisible, doctors do too. Managers don’t see or hear them dealing with toxicities that need expert input. Managers see drugs that the evidence says work well and are free of problems and figure doctors can be replaced with cheaper prescribers.
When you are faced with a patient who complains about being suicidal – the first scientific task is to work out what is happening. It’s a judicial task. Key to this is the conversation with the patient who may say ‘Doc I’ve been depressed and suicidal before but this was different – I think it’s the drug – it cleared when I lowered the dose’.
This engagement is a scientific engagement with the data – the person. If you conclude the drug has made the person suicidal but the published academic adverts do not seem to support this, the next scientific task is to reconcile the mismatch.
In the case of SSRIs and suicide, you find:
- companies breached FDA regulations in hiding events
- companies misapplied statistical methods
- the entire literature on these drugs is ghostwritten
- there is no access to the figures/people for independent scrutiny
- FDA don’t have the data
When you get to the bottom of this you find what your patient has told you squares with the real science you find behind the apparent science. This is not surprising if the answer to the question What is Data – is that in Clinical Trials People are the Data.
Slide 46
Another key date in the transition from What Used to Called Medicine to our modern Health Services was World Mental Health Day 2002. On this day, Newsweek ran a feature on Teen Depression – 3 million American teenagers have it. They will go on to become alcoholic, drug addicts, job failures, have marital breakdown and will suicide unless detected and treated.
Never fear though the US Cavalry is on the way – Prozac has been approved for children and as Newsweek knew Paroxetine/Paxil and Sertraline/Zoloft were about to be. That same day FDA approved Paxil for use in teenage depression.
Slide 47
The year before Paxil was approved, Study 329 appeared in the journal with the highest impact factor in Child Psychiatry – a trial of paroxetine v imipramine v placebo in adolescent depression. The article had an authorship line to die for. When people like this speak, you listen and obey – a bit like the Bible.
The woman who really wrote the paper whose name is Sally Laden is not listed there.
Slide 48

Study 329 actually finished in 1998. GSK looked at the data and concluded it was negative – the plan was to take the good bits of the data and publish them – as the Keller article.
This 6-page 1998 document was the basis for a Fraud case New York State took against GSK in 2004. To prove fraud you have to be able to prove intent – not just show that something wrong was done. The document demonstrates GSK’s actions were deliberate. GSK disowned it.
Slide 49
You heard me say FDA approved Paxil for children in 2002 but you know it’s not approved. Here is part of FDA’s approvable letter to GSK from October 2002 in which you can see that GSK told FDA that Study 329 was negative.
In response FDA agrees to approve paroxetine on the back of 3 negative trials and also agrees not to mention this.
Why would FDA do this? Well GSK may have said – you realise if you mention this trial was negative we might get sued for Fraud – as later happened.
Slide 50
Ten years later the US Department of Justice resolved an action with GSK over Study 329 and other trials for $3 Billion – then the most expensive resolution of a corporate case in history.
Slide 51
A few months later Andrew Witty, the CEO of GSK appeared on the front page of the BMJ – as St Andrew Witty. This is an Obama image. With an inside paean of praise.
At this point, the BMJ and GSK and Sense about Science were all part of a new initiative that may have been cooked up within GSK or Sense about Science – called AllTrials.
Sense about Science have a mission to defend corporate interests. In the case of medicine this means promoting RCTs, EBM and making sure that independent experts are mobilized to counteract anything that people like Healy might say. The media are more willing to tackle the Israeli Secret Service or the National Rifle Association than they are to investigate any drug or fertilizer issue that Sense about Science are linked to.
When SaS formed AllTrials in early 2013, GSK immediately jumped on board. And lots of bioethicists and good people donated money to AllTrials.
Slide 52
AllTrials did not mean AllData. It means controlled access to the data pharma wants you to see. This means they get to publish their ghostwritten articles and someone like André Marx can say yep he has seen the data – which makes the Fraud even more impenetrable.
Slide 53
But as a result of New York’s Fraud action, GSK agreed to post Company Study Reports on their website.
In 2013, Peter Doshi, one of the most remarkable people I know, proposed a – Restoring Invisible and Abandoned Trials – a RIAT initiative. In the case of an unpublished or incorrectly published trial, if there was access to the data researchers would write the study up as it should be written.
Thanks to New York State there was a lot of access to Study 329 material.
Slide 54
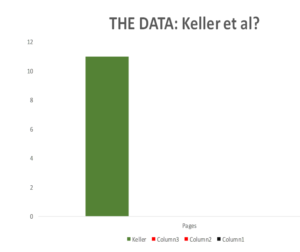
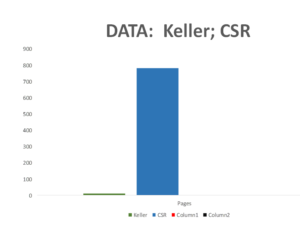
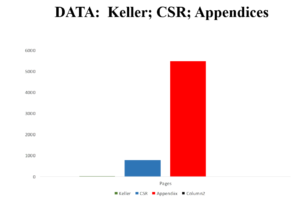
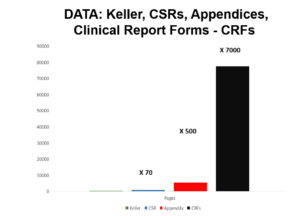
The original Keller paper is 10 pages long.
Slide 55
Companies normally send regulators Company Study Reports (CSR) on a trial – for Study 329 these come to around 800 pages. Until recently regulators saw much less than this and some still see even less.
Slide 56
Now the Study 329 CSR comes with Appendices A-H. GSK originally just put up the CSR not the appendices on their website. Peter Doshi asked New York State to require them to post the Appendices and they agreed to post A to G. This brings the total page number up toward 6000.
Slide 57
We applied to GSK for access to Appendix H. Maybe because 100,000 people work for GSK some right hand of one didn’t know what the left hand of another was doing but after several refusals we got access. This contains 77,000 pages or thereabouts.
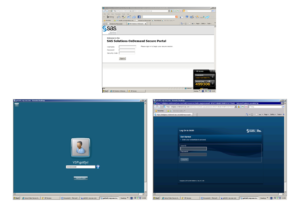
Slide 58
It was access through a portal with a Triple Lock. We could not download or print-off anything. And we got chucked off regularly but still after 9 months work on this we were able to come to a view about how to interpret the material.
Slide 59
The BMJ website says they take 2-3 weeks to accept an article and 8 – 10 weeks after that to publish research. A year after submission we were still not accepted. BMJ and their lawyers were too scared. There are usually two reviewers and one review process – we had 7 reviewers and 7 processes and a beyond belief twist to the story.
Slide 60
Over a year after submission we got published.
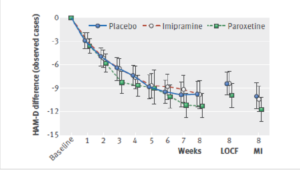
Slide 61
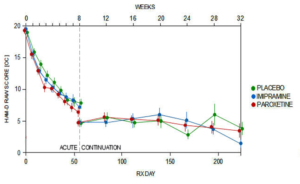
No matter which way they were analysed the figures, there was no benefit, as perhaps you’d expect. This was an 8-week trial with a never published 26-week continuation phase. These are the 8-week figures.
Slide 62
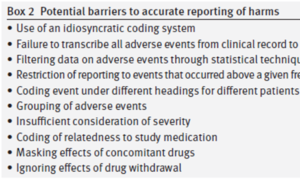
Adverse events are where the fun lay. Here are some of the differences between the original and restored versions we pointed to.
In the original
- Events went missing
- Statistics eliminated events
- Adverse Events were coded – first line of authorship
- Adverse Events were grouped – second line of authorship
- No account was taken of withdrawal
- Placebo patients could get an SSRI antihistamine
Once you’ve coded suicidal events as emotional lability, and grouped these events with headaches and dizziness under a neurological heading, the problems have vanished – leaving your ghost writer – Sally Laden – able with a clear conscience to talk about how wonderful the drug was.
Rather bizarrely, if you think about it, no methods section of any clinical trial paper ever deals with these key acts of authorship.
Slide 63
There has to be a coding system. This is the first line of authorship. It can cause its own problems – like the guy in the Pfizer trial ending up as a death by burns. This was not helped by the fact that GSK used a system no-one had ever heard of. Emotional lability is a coding term in the system they used and all the suicidal events got coded under this.
We used MedDRA which is the most commonly used system now – so you can go in an check all our codes and you may well not agree. We’ve invited GSK and everyone to suggest alternate codes for particular events.
This is a key point behind the study – no RCT should ever give a conclusive answer. Like all good science, a good trial will raise rather than answer questions.
Slide 64
Headache turned out to a key code. Headaches and dizziness were the two most common events in the trial. They might be grouped under neurological, or cardiovascular – remember imipramine was in this trial and it will lower blood pressure especially when given in 300 mg doses to teens when the usual adult dose is about 150 mg.
Grouping is the second line of authorship. Once you code and group you have close to written what the paper has to say. Yet no-one gets to see this – there are no methods for how coding or grouping is done, no oversight etc.
If you grouped all behavioural events like suicidality as neurological and added in headache and dizziness there was no obvious difference between the paroxetine and placebo groups.
This Headache book is authored by Elizabeth Loder – the research editor for BMJ. She was the person who kept moving the goalposts on us. She was very agitated about everything we did especially with headaches. We googled her and found she’s a headache expert.
All throughout she had accused us of various conflicts of interest and animosity to GSK. We found her husband worked for Ropes and Gray – a law firm defending GSK in the Department of Justice case that cost them $3 billion. She had not declared this conflict.
A year after we submitted the article, her conflicts came to light and BMJ capitulated.
Slide 65
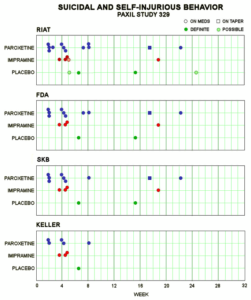
On this slide, at the bottom you see the suicidal events in the original Keller paper.
Then in 2003 GSK were asked by FDA to look again at their data and this is what you find – an increase in events but not statistically significant – so nothing to worry about they said. The increase isn’t really there.
Finally, on top you see what we found.
Slide 66
What you don’t see on this slide was the 4 teenagers who dropped out with intercurrent illness – like the 15-year-old boy – we only discovered this afterwards. So there are potentially 4 more events to add to the previous slide. The dropouts were all taking Paxil and at least one of them was a significant behavioural event – so things are even worse than we thought and than GSK let FDA know.
Slide 67
What no-one has seen is the data from the continuation phase – but you see it here. I should tell you at the end of the acute phase a very large number of placebo patients dropped out – with rating scales scores that were the lowest – that is they seemed really well. They aren’t in here. Without them you see placebo does pretty well compared to active drug. With them left in placebo would likely have been the best.
Slide 68
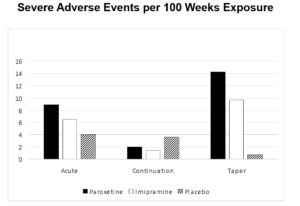
Having the continuation phase data made it possible to look at Withdrawal Events. The withdrawal period was the most likely to produce suicidal events. When this trial was done SmithKline Beecham were denying there were any problems with withdrawal on their drug.
Slide 69
You can find everything about this trial on Study329.org – things like the approvable letter from FDA, along with the document used to file a Fraud charge against GSK.
It’s worth noting that GSK’s behaviour here was not exceptional. Study 329 was a good trial. What you see here is standard practice for all trials by all companies for all drugs.
FDA’s behaviour was not exceptional. It’s standard practice for all drugs – up to one third of the articles on antidepressants claim the drug works well and is safe when FDA internally have decided the study was negative. But FDA say nothing.
We now know the entire literature on antidepressants for children is ghost written and there is a total mismatch between what the literature claims and the data says
Ah but you might say Prozac works for children. No, it doesn’t. The Prozac trials that led to its approval were negative too – and FDA knew this.
Slide 70
You can also find out all about the story in this book Children of the Cure. This includes material like who was sleeping with who and who really found the documents that I’ve mentioned and broke the story open, which I haven’t told you here.
Slide 71
So we have a paradox. There are now 45 RCTs of antidepressants in children – all 45 are negative. RCTs were supposed to stop therapeutic bandwagons. So, nobody should be giving these drugs to children.
Greta Thunberg’s generation are very concerned about the chemicals we are pouring into the environment but not it seems about the chemicals they pour into themselves. Antidepressants appear to be the second most commonly taken drugs by teenage girls. Adolescents will trample all over a doctor who hesitates about handing them over.
Slide 72
This is not down to pharmaceutical marketing. There has been a change in culture. This seems to be a generation who believe more in technologies than relationships – especially in healthcare.
Something about our world makes it harder to live with thinning hair if you are male, mild acne if you are male or female, or negative emotions in you are anyone of any age.





















more revealed and the Fid hypothesis theory…
Posted on Thursday 11 October 2012
http://1boringoldman.com/index.php/2012/10/11/more-revealed-and-the-fid-hypothesis-theory/
a GSK watcher of note] …
With Transparency Pledge, Glaxo Makes Promises No Other Drug Company Has
Forbes
by Matthew Herper
10/11/2012
In an unprecedented move that could signal dramatic changes in the drug industry, GlaxoSmithKline is promising to make detailed data from its clinical trials available to independent researchers so that scientists can draw their own conclusions about the safety and effectiveness of its new drugs.
What Witty is saying he will do now goes way beyond anything even Eliot Spitzer asked for. Glaxo would put in place a system by which independent researchers could request the data about what happened to individual patients in its clinical trials, and would be granted access if an independent group of experts thought the idea had scientific merit
One obvious detail to worry about: the panel of experts, convened by Glaxo, who will decide which independent scientists get access to the company’s data. Will the panel allow critics, like Nissen, to look at this patient-level data, as the this information is known?
“We’re not asking that panel to make judgements on the value of the questions being asked,” says Vallance. “We’re asking that panel to take a view on the scientific validity. So the answer to that question is yes, someone like Steve Nissen would be able to access patient-level data if he had a protocol that would answer a scientifically valid question robustly.” Vallance also warned, however, that it would be “anti-public health” for researchers to trawl the data for side effects without clear questions, because it would lead to unwarranted side-effect scares. But he said that even in the case of Glaxo’s best-selling asthma drugs, which some doctors warn can sometimes hurt patients, what he wants are the real answers. “We are not looking to hide something about our medicines,” says Vallance. “If it is a scientifically valid question, answered robustly and comes up with an answer, I think we need to know that answer.”
A timeline that suggest that this hypothesis might be elevated to the level of theory:
“New York Attorney General Eliot Spitzer sued Glaxo in 2004 for not disclosing negative data that linked Paxil to suicidal thoughts in children. As part of a settlement, GSK agreed to post all the results of its clinical trials on a company Web site.”
GSK complied with a partial posing of the data in 2004, summary data rather than patient level data
August 2, 2012: “When I scrolled down, it had changed. There was Study 329, the famous one [Efficacy of Paroxetine in the Treatment of Adolescent Major Depression: A Randomized, Controlled Trial], but after all the narrative summaries, it had a list of Appendices [Appendix A through H]. I’d never seen them before and as I opened them, I realized that what I was seeing was the raw data – HAM-D scores/Subject/Item/Week and all the other various rating scales. The page said ‘Updated on Thursday 2 August 2012‘ down at the bottom.”
a GSK watcher of note] …
So I wrote everyone I could think of who were onto the Study 329 story asking about the change, and I was told by Dr. David Healy [author of Pharmageddon] that Peter Doshi had noticed that it was missing and had alerted the NY AG that the Appendices [Data] weren’t posted. It was apparently part of an order in the Paxil suit in NY [settled in 2004] that they post all the data. And after a bit [6 months or so], there it finally is.
http://1boringoldman.com/index.php/2012/08/21/a-movement/
So, back to Paxil Study 329. Peter Doshi noticed that the raw clinical data from GSK’s Paxil Study 329 was missing from the web site above [Paroxetine and pediatric and adolescent patients] and questioned whether it was fulfilling the settlement requirements from the old 2004 NY GSK suit. I don’t know the details of how it came to be, but here on August 2, 2012, the data has finally been posted [after another $3B suit just settled against GSK also related to this study] – 11 years after the publication of the article and 8 years after data transparency was prescribed by the New York settlement. Better late than never I suppose, but way earlier would have been infinitely preferable.
“All Drs I saw afterwards were of the opinion the drug could not have done this.”
watcher of note] …
recovery&renewal Retweeted
Katinka Blackford Newman @antideprisks
antidepressantrisks.org/stolen-lives/sally…
@davidhealy @Reduxreloaded
https://www.antidepressantrisks.org/stolen-lives/sally
of note] …
OPEN DEMOCRACY HOME PAGEt
(It doesn’t expalin what is to be done with the data – would be usefult o pharma companies for one – Tell us the secret Javid -it’s not nice to treat the population just as data/research fodder .
UK government could face legal action over huge, secretive health database
Exclusive: Lawyers acting for openDemocracy have written to health secretary Sajid Javid demanding transparency over database originally run by Palantir ast month the government ended a controversial deal to handle adult social care data with US ‘spy tech’ firm Palantir.
This data is instead set to be held in a new system called Edge, which will be run internally by the Department of Health and Social Care – but built by British defence contractor, BAE Systems.
Caroline Molloy
11 October 2021, 2.56pm