
This is part 3 of a set of lectures organized by André Marx, a family doctor from Stockholm. Its alternate title is Girl who Eats Salt. This follows Girl with a Clinical Trial Tattoo, and Girl who Tackles Ghosts.
A video recording done by Thomas Hultgren of Girl who Eats Salt is Here.
Our lady is still in the waiting room. She will come in before the end of the lecture.
Slide 74
This is the Menai Bridge, the oldest suspension bridge in the world. Seen from the side it is probably Wales’ most famous view. You are looking from the UK mainland side over at Ynys Mon, the Isle of Anglesey. I used to live just over here. Walking to and from work I crossed this bridge thousands of times.
It opened a century before we had cars. But it was engineered well and two centuries later the bridge can be full of heavy trucks and coaches of tourists and can take these no problem.
The message of randomized trials – RCTs – and evdence based medicine – EBM – is we now have engineered medicine so that just as Menai Bridge is a long way from the rope bridges we used to have, so also health services are a long way from What We Used to Call Medicine.
Slide 75
But Medicine is and always has to be a matter of rope bridges. It cannot be engineered the way some fantasize.
Since EBM, health services have become increasingly dangerous. We have let the slats on the rope bridge rot and some fall out without being replaced. Missing slats, unreported negative trials are bad enough, but negative trials reported as postive – rotten slats – are even more dangerous.
Even before Covid life expectancy was falling. We know increased drug consumption shortens lives, increases hospitalizations and reduces quality of life. We know that reducing medication burden can improve all of these but we don’t do it.
Slide 76
Another way to put it is this. EBM and RCTs give us a top-down approach to medicine – a shower approach. Adverse events require a bottom-up medicine – a bidet approach. We need both – but when we are at our most vulnerable, as infants or old and frail or injured, if you have to choose one only bidets or bottom up washing is more important. See Life is a Bidet Old Chum.
Earlier I said there are two questions – What is Data and What are RCTs. The answer to the first is that People are the Data in medicine. If you don’t have access to the people, you are not doing science. At the moment the only science we have is in Case Reports.
Slide 77
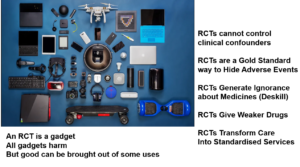
The answer to the second question is RCTs are a gadget. They aren’t knowledge. They are algorithmic – they try to exclude you and your patients’ judgments as to what is happening. They figure the truth will emerge from bytes.
- RCTs cannot control clinical confounders
- RCTs are a gold standard way to hide Adverse Events
- RCTs generate ignorance about medicines
- RCTs give us weaker drugs
- RCTs are central to the move away from HealthCare to health services
Slide 78 and 79
These gadgets are used to hypnotize us. The three elements of any hypnosis involve stimulus binding:
- If X then Y – in this case drugs are an answer to the figures from rating scales, peak flow rates or bone densities. Sure, maybe there is nothing much wrong with you but what could be wrong with making the figures a little better.
- Adverse events risk breaking the trance – but you can deepen the trance if you persuade patients and doctors their agitation on an SSRI is a sign their depression is getting worse and needs more drug or they have bipolar disorder and need mood stabilizers – once they take this step they are yours forever.
- Diminished personal contact
- No room for discretion
Slide 80
This is not a pharmaceutical company plot. Left wing parties have done even more to build the trap we are now in than the Right Wing – they are even more technocratic.
In 1998 the British Labour Party introduced NICE guidelines and then in 2002 a plan for the NHS which involved screening everyone’s blood sugars, lipids, blood pressure, peak flow rates and bone densities. The idea was to give everyone the same Quality treatment.
For every set of figures that are slightly out of joint, a drug becomes the irresistible answer – particularly if doctors get a bonus for prescribing.
As mentioned, we can have a free Health System if we primarily do things that save lives and get people back to work, but prescriptions are now mostly given to healthy people. This can only increase disability and take people away from work.
Slide 81
The idea of treating risks rather than diseases emerged in medicine in the 1980s before anyone had heard about Ulrich Beck and Risk Societies. Beck pointed to the risks in the technologies we were surrounding ourselves with and Germany took this on board and pushed back against nuclear power – environmentalism became a much stronger force
In health, perceptions of risk have not led to a pushback against technologies. Instead, we have embraced technologies and the risks of these technologies have vanished almost entirely.
Slide 82

Pharmaceutical companies have cleverly exploited this. Selling the idea that treating the risks of heart disease is cost effective in the way saving a life after a heart attack is. The system will save money.
Faced with complaints about high-cost drugs, companies say the drug bill remains a constant 10% of overall health spend. But if drugs work, health spend should fall and drugs should be a greater proportion of it. Our problem now is we don’t only pay a high cost for drugs, we increasingly pay for a sales force for drugs.
We pay for screeners to do bone scanning which leads to bisphosphonate prescribing, which leads to more fractures. So we pay the hospital costs of these fractures and other adverse events. There are now more admissions for bisphosphonates than osteoporosis and it is same for admissions with diabetes drugs and mental health drugs. In addition, we employ auditors to track what is happening – why are there more admissions when there should be less. And managers to make sure everyone is keeping to guidelines – because of course its doctors not keeping to the guidelines who are the problem.
This lets pharma claim their drugs remain 10% of the budget. The drugs are actually costing us more and more for which we get less and less. This is a game that is win-win for pharma – the more drugs given to healthy people, the more admissions to hospital which mean the drugs budget will always be 10% of overall health spend no matter how costly the drugs.
Pharma are not particularly skilful game-players. Should we blame pharma for playing the game or doctors and politicians for being so pathetic?
Slide 83
If we give drugs to people who are ill and save their lives and get them back to work we can have Free Universal Healthcare. Instead we are moving rapidly toward industry’s vision of Universal Health Coverage – everyone on drugs or vaccines – which will bankrupt us.
Slide 84
The Labour plan for the NHS in 2002 envisioned doctors being replaced by nurses. In 2004 the front page of this US magazine sees the same future in store for doctors. For you.
Slide 85
We are moving into a supermarket type health service. Nurses and pharmacists will be at the key points doing the scopes and issuing prescriptions.
Doctors will be like the supervisors marching up and down ready to answer queries should one arise – but with they will be in closely defined roles.
Your pet will likely get more person centred care from a Vet than you will these days from a doctor.
Slide 86
The managers are off site writing flowcharts for how the algorithms fit together. Medicine used to be about minimizing risks for your patients. Your health service job now is about adhering to processes aimed at minimizing risks doctors and their patients pose to the organization.
If something doesn’t fit into the process like a patient dying from an adverse event, a root cause analysis of the event will take place and say the system ticked all the boxes.
For the system to do anything else, there would have to be a box in the flowchart that recognises that adverse events can happen because the medical literature is fake. That would require a second box explaining how a fake literature would be managed – and not even Stefan Lofren the prime minister of Sweden, or Jacinda Ardern in Neew Zealand, has the authority, or cojones, to put a box like that in place.
Medicine, as it used to be called, aimed at bringing good out of the use of a poison. This does not compute for managers, or insurers, or regulators, or politicians.
Slide 87
For doctors to practice medicine rather than be health service supervisors of nurses – they would have to retain their saltiness. When salt loses its saltiness it’s no good for anything.
Slide 88
We are now training low-salt Doctors to be supervisors, passionate about appraisal, assessment, following processes and ticking boxes rather than physicians engaging with twentieth century things like adverse events.
Slide 89
In psychiatry, we were great at telling people to take responsibility for their own lives – while not being very good at living our own lives.
Slide 90
To save medicine we need to take responsibility. Our literature could not be ghostwritten if we were taking responsibility. Companies could not hide the data from us if we were taking responsibility. It’s our job to police the medical literature.
We need to take responsibility at inquests and pay no heed to medical insurers who tell us to never agree the drug caused a death. Insurers are supposed to be supporting medical practice but in fact they support pharmaceutical companies.
Slide 91
We need to follow the example of Lysistrata who persuaded Greek women they could stop a war by refusing to let their men make love to them until the war stopped.
Pharma needs us more than we need them. If we refuse to prescribe their drugs without the data, they will have no option but to hand it over – and drugs will become cheaper when everyone gets to see the hazards.
Slide 92
Why is it do you think that we report so few adverse events on drugs? 1 in 100 it seems. And why do regulators pay no heed to our reports. Pilots also have adverse event reporting systems. If no one pays heed to the hazards they report, pilots would stop flying – so the system pays heed.
In plane flights, getting to NYC 15 minutes quicker is not viewed as important as getting there alive. Safety is more important than effectiveness. Pharma has persuaded us we want to get places 15 minutes earlier – and in some cases provided fraudulent data that their pill will do it for us.
Slide 93
Regulators pay no heed to our reports. It’s not their job to heed them. They file them away. No regulator ever tries to work out if a drug has caused a problem. The Swedish or EMA regulator never decides if a drug is causing a problem.
Regulators are bureaucrats. They are not trained to make clinical judgments. The few who were clinically trained are now bureaucrats because they don’t like meeting the kind of person in the waiting room. Or they may have been an orthopedic doctor who is now being asked to give a view about contraceptives. They have no access to the data or way to check what really happened. And they are acting in an agency which is obliged to partner industry.
There is one thing regulators do which is very important. Important for pharmaceutical companies. They remove your name from any report.
Slide 94
The execution of Walter Raleigh in 1618 shows you what needs to be done to change this. Raleigh was executed on the basis of hearsay – people saying things about him who were not prepared to come into court and be cross examined. You have to be able to cross examine someone in order to know what weight to put on their testimony. After his execution, legal systems worldwide introduced a hearsay rule – evidence from people without names is not taken into account.
There are thousands of reports of PSSD with EMA or FDA. If you get PSSD and take an action against a doctor or company, and tell the court there are thousands of reports of this, their lawyer will jump up and say ‘Your Honor, this is all hearsay’. The Court will then block any mention of these reports.
To change this both you and your patient need to report and insist your names are left on the reports. Better again send named reports to the pharmaceutical company who market the drug. Companies are legally obliged to follow you up and work out if their drug caused the problem. Regulators are not.
We probably will have to have another repository of these reports. Perhaps something like RxISK.org
Slide 95
But this will take courage, and a heart and brains. You have to understand you are committing to taking on the scary Wizard of Oz. You are committed to going to Court, committed to having a pharmaceutical company come and interview you. Committed to going against the advice of your medical insurer who will say you should not blame the drug.
But don’t worry. You won’t end up in Court. Once you make this commitment, the curtain will drop and everyone will be able to see the fraud behind it.
Slide 96
Donald Trump weaponized the phrase Fake literature for me. He gave me another good idea also. After a school shooting in the US, he said we need a good guy with a gun outside all these schools. People were horrified at the idea. But had no easy answer for the follow up – well if a good guy with a gun isn’t a good idea why do we have them outside the White House?
Drugs are like guns – they can be very helpful in certain situations. But we know that letting them multiply up and leak into every situation is a recipe for problems.
Slide 97
Drugs and Guns are techniques – gadgets. All gadgets create an arms race. Whether the use of these gadgets enhance or diminish us is down to us – but if we stop thinking about what we are doing when we use them we are highly likely to be diminished.
RCT gadgets mean the drugs arms race is not about creating more effective chemicals – it’s about creating more effective propaganda. Almost by definition this diminishes us.
The guns arms race brings out an important message. Efficacy has its limits. We can make weapons more and more effective to the point that they can’t be used and as a result the Vietnamese and Aghans can beat the Americans.
Slide 98
The same holds true for drugs. If you are on more than 3 medicines, effectiveness diminishes. To get the most effectiveness you need to be on 3 or less.
Two years ago, 40% of over 45s are on 3 or more drugs every day of the week – this figure includes the people who never come to see doctors – so even more of those doctors see will be on this number of drugs. 40% of over 65s are on 5 or more drugs every day of the week.
And we know that life expectancy even before Covid was falling. We know that reducing medication burdens can increase life expectancy, reduce hospitalizations and improve quality of life.
Paring our rapidly escalating medication burdens back to 3 or less should mean taking the values of the patient into account – in terms of quality of life as well as length. But we are not doing it.
We introduced RCTs so that efficacy could be used to improve safety. Which hasn’t worked – it has produced just the opposite outcome. Now there is a real chance to improve safety by focussing on efficacy in a different way – by deprescribing in order to get efficacy.
Slide 99

Our lady has come into the room. What is it she wants? She is a tough woman, but still she is nervous that her doctor will be hostile. She is going to tell you she has a problem with a drug you gave her, and she knows you wll bristle.
She has seen at inquests for family members killed by drugs that you betray bereaved families when they need you most. You might express sympathy, but you never say the drug you gave her someone’s son killed him. You are advised by your medical insurer never to say the drug caused the problem – it is always the disease that kills as Eli Lilly said in 1991.
If you betray her at a public inquest, how much nastier are you likely to be in a quiet office where no-one can see or hear you?
One mother whose 15-year-old daughter with no mental health history recently committed suicide after taking Accutane for Acne said about inquests – that it would be better if her daughter had been murdered because at least that way she might be able to meet the perpetrator in Court.
Medicine is now full of crimes with no perpetrators.
Slide 100
She knows you are only being nasty because you too are scared. If you publicly admit a drug can cause a problem, you fear the regulators and guideline makers, and your colleagues and politicians and the media will be nasty to you. Even Greta Thunberg’s generation will mobilize against you. So, you’re passing the pressure on you on to the woman who has come with a problem.
Patients with PSSD often go on to commit suicide. It’s partly because of the horrific problem. It’s partly also because of the ridicule they get at from doctors. You tell them they will remain ill for ever if they research things on google. You ask how could a drug that has left their body years ago still be causing problems? You suggest they might need an antidepressant.
Slide 101

In this story, as in the Arthurian legend it is based on, once you listen to this woman and give her control of her own life, you bring out the best in her. She or he can become the most interesting and, in that sense, attractive people imaginable.
Rather than a problem for you, the people in the waiting room could be free research assistants making your job much more interesting if you just weren’t so commigtted to being the expert and bristling at any hint it might be otherwise…. I have a PhD in the serotonin system but have learnt more from patients googling things about the serotonin system than I ever knew.
Our lady wants your help to live the life she wants to live. There may be a disease that needs treating – but this is important in so far as it impacts on the way she wants to live her life. She doesn’t want you to tell her how to live a life – she may be doing better at that than you or I.
What’s at stake is that you can have 100 heartsink patients or 100 free researchers helping you discover things you never knew about. Your job could be fun.
I’ve talked about the courage needed to change things by putting your name to adverse events. I’ve talked about a smart way to do things by getting safety in through the back door by deprescribing. But there is another central element. Its something very strange and hard to put in words – you have to like people. There is possible virtuous cycle here if you like someone they become more interesting and you like them and others more, which leads them to like you too because you like them.
Someone who thinks they possess absolute truth does not like people.
Slide 102
I have outlined some problems in this talk. The full story of how we got here is in Shipwreck of the Singular. The references to this are online on Samizdat.org – they include not just details of the articles and books and interviews but many of these articles and books can be downloaded, as can over 100 interviews with key players in this history.
There is also a dhblog.wpengine.com blog which has a Politics of Care forum where this lecture will appear.
And RxISK.org where people harmed by drugs can report their problems on treatment.
If any of you want to chase these issues further, please feel free to contact me on david.healy@rxisk.org
Slide 103
Perhaps you can now see why I find this quote from Vaclav Havel so compelling – it was shortly before the Velvet Revolution.
One word has been changed. The Word Health on the first line. Havel said Europe.
A Spectre is haunting Health… the spectre of Dissent
You do not become a ‘dissident’
just because you decide one day to take up this most unusual career.
You are thrown into it by your personal sense of responsibility…
You are cast out of the existing structures and placed in a position of conflict with them.
It begins as an attempt to do your work well
and ends with being branded an enemy of society. …
The dissident is not seeking power.
He has no desire for office and does not gather votes.
He does not attempt to charm the public.
He offers nothing and promises nothing.
He can offer, if anything, only his own skin—
He offers it solely because he has no other way of affirming the truth he stands for.
His actions simply articulate his dignity as a citizen, regardless of the cost.
Vaclav Havel, The Power of the Powerless, 1978























I think this is a most powerful and moving post and hope at least some of the attendees will take up the call to arms
This is an example of why I would never disclose adverse events to Bayer Pharmaceutical or any other and why I hope Rxisk will be setting up something independantly which can be used world wide for the individuals’ benefit as well for the general good rather than the benefit of Bayer
Privacy Statement for Pharmacovigilance Data
Bayer takes product safety and your privacy seriously
Our Pharmacovigilance obligations require us to process certain information, which allow to directly or indirectly identify a person, (“Personal Data”) of a patient and/or the reporter of an adverse event that we receive in order to comply with strict obligations to perform benefit-/risk assessments of Bayer Health Products continuously and report suspected adverse reactions or events to relevant regulatory authorities.
This Pharmacovigilance Privacy Statement (“Statement”) provides important information to you about how we process Personal Data for PV purposes, in line with our obligations under applicable data privacy laws and in particular the EU General Data Protection Regulation ((EU) 2016/679) (“GDPR”).
If you are a resident of California or Nevada in the United States, you may have additional rights regarding your personal data. found here.(These are described disengenuously as ‘exceptions’ to other states but allow Bayer to process much more personal info)
He is considered by some to be one of the most important intellectuals of the 20th century.
Havel first rose to prominence as a playwright in works such as The Garden Party
What about the pharmacy data that’s already being used by PHARMA to track physician’s prescribing patterns? And where are the departments of pharmacology and the schools of pharmacy in following medication efficacy and safety? or the HMOs? or the Health Plans? the VAH? What about the waiting room questionnaires? I’d much rather they ask about the medications the patient is on than being used to screen for depression. It’s really the ongoing data after a drug is in use that clinicians need anyway – more important than the RTC that gets things started.
So while it’s important to continue the push for data transparency and clinical trial reporting reform, it’s also time to explore other ways of gathering and evaluating the mass of information that might free us from the commercial strangle-hold we live with now – and potentially give us an even better picture of what our medications are doing over time.
There’s a way out of this conundrum. The task is to find it…
the commercial strangle-hold…
Posted on Sunday 22 January 2017
http://1boringoldman.com/index.php/2017/01/22/the-commercial-strangle-hold/
And if we find it…
https://www.youtube.com/watch?v=wQTpmhmQCS4
Michael P. Hengartner, PhD
@HengartnerMP
In sum, the ANTLER trial raises more questions than it answers. Although there were more relapses in the discontinuation group than in the maintenance group, the effect of withdrawal symptoms was not taken into account. Unblinding was also frequent and was not taken into account.
Melissa Raven
@psychepi
re Lewis et al. 2021 ‘Maintenance or Discontinuation of Antidepressants’ Thread by
@markhoro
on Thread Reader App
https://threadreaderapp.com/thread/1443505742842109953.html
Startling lack of interest in confounding by withdrawal symptoms in this antidepressant discontinuation study in NEJM: bit.ly/3AYzP2Z Withdrawal symptoms in this discontinued group render this study uninterpretable. (1/n)
The Garden Party…
“the other good news is that if you do want to come off antidepressants for whatever reason, this can also be done safely over a couple of months.” – Professor Sir Simon Wessely –
No Time to Die…
“I’m gobsmacked,” says @markhoro, who is also, interestingly, at @UCLPsychiatry . “This study is so against the grain. It seems to ignore all the work of the last couple of years, and the explosion of awareness of the trouble people have in #comingoff #antidepressants.”
Miranda Levy
@mirandalevycopy 3h
Last week, we saw an astonishing study about how “long-term antidepressant use is beneficial.” Here is my riposte in @Telegraph, with the fabulous help of @markhoro and @Peter Gordon.
I’m afraid it’s behind a paywall, but you can register for a free trial.
https://telegraph.co.uk/health-fitness/mind/antidepressants-life-should-taking-first-place/
Miranda Levy
@mirandalevycopy
·
3h
@DrDavidHealy @stimimi
If antidepressants are for life, should you be taking them in the first place?
A new study goes against the scientific grain by suggesting that long-term use of pills can be beneficial, writes Miranda Levy
ByMiranda Levy3 October 2021 • 12:00pm
https://www.telegraph.co.uk/health-fitness/mind/antidepressants-life-should-taking-first-place/
Great article by Miranda Levy, should be read by all – I registered for my free ‘trial’ –
Expert reaction to study on long term use of antidepressants, discontinuation and relapse
https://www.sciencemediacentre.org/expert-reaction-to-study-on-long-term-use-of-antidepressants-discontinuation-and-relapse/
‘chumocracy’ in action – Aye, Peter – the bright young bucks leave them standing…
Featuring the first major in-depth broadcast interview with Sir Patrick Vallance
Government Chief Scientific Adviser, and the 10th anniversary of The Life Scientific
Tuesday 12 October, throughout the day on BBC Radio 4
As Chief Scientific Advisor to the government during the pandemic, Sir Patrick became a household name. His calm, clear summaries of the state of our scientific understanding of the virus were welcomed by many across the country, and the value of science, and scientific advice, were thrust centre stage. Now, as head of the new Office for Science and Technology Strategy, Sir Patrick plans to put science and technology at the heart of policy making in government.
https://www.bbc.co.uk/programmes/m0010gkl
The Life Scientific
3 Nov 2015
Patrick Vallance on pharmaceuticals
While GSK is no stranger to scandal, since he joined, Patrick has attempted to tackle the culture of secrecy
https://www.bbc.co.uk/programmes/b06mdbnq
17.46You and your CEO Andrew Witty took another radical decision joining Alltrials
20.42 When researchers got access to the trial data regarding the controversial antidepressant drug Seroxat
“It’s inevitable that side effect things will occur from time to time and frankly I’d rather know them than not know them” …
“I’d rather know, I’d rather get it out there” …
https://study329.org/correspondence-with-gsk/
2013-06-14 — The RIAT team sends an email to GSK, Sir Andrew Witty (CEO) and Patrick Vallance (President of Pharmaceutical R&D), notifying them of the RIAT article publication and requesting that if they plan to restore any old GSK trials, they respond as soon as possible.
From: Peter Doshi
Date: 14 June 2013 at 19:26
Subject: Restoring Invisible and Abandoned Trials – your response requested (GlaxoSmithKline)
Dear Christopher Gent, Andrew Witty, and Patrick Vallance
‘side-effect ‘things’ …
addition to info collected by Bayer (above)Effective Date: July 1, 2021
This Privacy Statement Addendum provides additional information for residents of California or Nevada in the United States regarding what personal data we collect about you, what we do with it, and how you can control it in connection with our websites, mobile apps, or other online services (the “Site”).
CALIFORNIA PRIVACY RIGHTS
Categories of personal data we collect
We collect information that identifies, relates to, describes, references, is reasonably capable of being associated with, or could reasonably be linked, directly or indirectly, with a particular California consumer or household (“personal data”). In particular, we have collected one or more data types in the following categories of personal data from consumers within the twelve (12) months preceding the effective date of this Privacy Statement:
Category
One or More Data
Types Collected?
A.
Identifiers such as a real name, alias, postal address, unique personal identifier, online identifier, Internet Protocol address, email address, account name, social security number, driver’s license number, passport number, or other similar identifiers.
Yes
B.
Any categories of personal data described in Cal. Civ. Code § 1798.80(e), such as name, signature, social security number, physical characteristics or description, address, telephone number, passport number, driver’s license or state identification card number, insurance policy number, education, employment, employment history, bank account number, credit card number, debit card number, or any other financial information, medical information, or health insurance information.
Yes
C.
Characteristics of protected classifications under California or federal law.
Yes
D.
Commercial information, including records of personal property, products or services purchased, obtained, or considered, or other purchasing or consuming histories or tendencies.
No
E.
Biometric information, for example imagery of the iris, retina, fingerprint, face, hand, palm, vein patterns, and voice recordings, from which an identifier template, such as a faceprint, a minutiae template, or a voiceprint, can be extracted, and keystroke patterns or rhythms, gait patterns or rhythms, and sleep, health, or exercise data that contain identifying information.
No
F.
Internet or other electronic network activity information, including, but not limited to, browsing history, search history, and information regarding a consumer’s interaction with an Internet Web site, application, or advertisement.
Yes
G.
Geolocation data.
Yes
H.
Audio, electronic, visual, thermal, olfactory, or similar information.
Yes
I.
Professional or employment-related information.
Yes
J.
Non-public education information.
No
K.
Inferences drawn from any of the information identified in this subdivision to create a profile about a consumer reflecting the consumer’s preferences, characteristics, psychological trends, predispositions, behavior, attitudes, intelligence, abilities, and aptitudes.
Yes
In particular, we collect the following types of information
Information you provide. We may collect your first and last name, email, street address, state, zip code, age, gender, demographic information, preferences, or other information you voluntarily send to us through the Site or through communications with us
Information gathered automatically. When you visit the Site, we may automatically collect information about your device or internet activity directly or through our third-party analytics providers. We (and third parties acting on our behalf)
We do not currently respond to those signals. Instead, we collect, use, and share information as described in this Privacy Statement regardless of a “do not track” choice.
Location-based information. When you access the Site, we may collect information related to your location through your device’s GPS, Wi-Fi, Bluetooth, or IP address.
Information from social media and other sources. We may collect information about you from other sources such as our affiliates, public and third-party databases, and other third parties. We may also collect information when you interact with us through social media. And if you link our Site with a social media account, then we may collect certain information related to that account.
Categories of sources of personal data
We obtain the categories of personal data listed above from the following categories of sources:
Directly from our consumers (for example, information provided through the Site, hardcopy forms, or other communications with us).
From consumers’ activity on the Site (for example, Site visit information collected directly or through third-party analytics providers).
From a device’s GPS, Wi-Fi, Bluetooth, or IP address.
From social media, marketing partners, and other sources (for example, affiliates, public and third-party databases, and other third parties).
Our business and commercial purposes for collecting personal data
We use the personal data we collect for our operational purposes or other purposes set out in this Privacy Statement. Those purposes may include:
Auditing related to a current interaction with the consumer and concurrent transactions, including, but not limited to, counting ad impressions to unique visitors, verifying positioning and quality of ad impressions, and auditing compliance with this specification and other standards.
Detecting security incidents, protecting against malicious, deceptive, fraudulent, or illegal activity, and prosecuting those responsible for that activity.
Debugging to identify and repair errors that impair existing intended functionality.
Short-term, transient use, provided the personal data is not disclosed to another third party and is not used to build a profile about a consumer or otherwise alter an individual consumer’s experience outside the current interaction, including, but not limited to, the contextual customization of ads shown as part of the same interaction.
Performing services on behalf of the business or service provider, including maintaining or servicing accounts, providing customer service, processing or fulfilling orders and transactions, verifying customer information, processing payments, providing financing, providing advertising or marketing services, providing analytic services, or providing similar services on behalf of the business or service provider.
Undertaking internal research for technological development and demonstration.
Undertaking activities to verify or maintain the quality or safety of a service or device that is owned, manufactured, manufactured for, or controlled by the business, and to improve, upgrade, or enhance the service or device that is owned, manufactured, manufactured for, or controlled by the business.
We also collect personal data about you for the following reasons:
For our internal efforts to operate and improve our business.
For advertising and marketing purposes.
For our general administrative purposes.
For social-media engagement.
For security purposes.
For other legal purposes.
For communication purposes, to get in touch with you in connection with any of the reasons discussed above.
In the twelve (12) months preceding the effective date of this Privacy Statement, we have disclosed one or more data types in the following categories of personal data of California consumers for a business purpose:
Category A: Identifiers.
Category B: Categories of personal data described in Cal. Civ. Code § 1798.80(e).
Category C: Characteristics of protected classifications under California or federal law.
Category D: Commercial information.
Category E: Health or biometric information.
Category F: Internet or other electronic network activity information.
Category G: Geolocation data.
Category H: Audio, electronic, visual, thermal, olfactory, or similar information.
Category I: Professional or employment-related information.
Category K: Inferences drawn from other personal data.
We also use the personal data we collect for our commercial or economic purposes, such as attracting consumers to buy, rent, lease, join, subscribe to, provide, or exchange products, goods, property, information, or services, or enabling or effecting, directly or indirectly, a commercial transaction.
Categories of third parties with whom we share personal data
We may share your information:
With our affiliates and other companies in which we have an economic interest or ownership rights.
With others working for us, which includes third parties that perform services on our behalf such as running our Site, sending communications for us, and executing marketing programs and promotions.
With other third parties, such as law enforcement or other government entities: (1) if we believe there has been a violation of our Site policies; (2) if we believe that someone may be causing injury to our rights or property, to other users, or to anyone else; (3) to provide information to law enforcement; or (4) as required by law.
As part of a corporate restructure, such as a liquidation, reorganization, merger, sale of the company, consolidation, change of control, asset or stock acquisition/disposition, or other corporate combination. If that happens, we will use reasonable efforts to make sure the transferee uses your information in a manner consistent with this Privacy Statement.
With third-party analytics providers, such as Google Analytics, Flurry, Adobe, or Crazy Egg, to collect and analyze information about your use of our Site.
People in psych hell holes are forced, not just coerced onto to drugs that cause suicide ideation, suicide and violence. She is lucky just to lose her job and not her life:
https://www.bitchute.com/video/5dCjnCtFqcHu/
Karen Kingston zeros in
https://rumble.com/vnhqap-receipts-dod-joint-artificial-intelligence-center-monitoring-vaxx-deaths.html
Heart sink doctors
OPEN ACCESS PEER-REVIEWED
Published: October 4, 2021The rise in opioid prescribing in primary care represents a significant international public health challenge, associated with increased psychosocial problems, hospitalisations, and mortality.
The intervention, the Campaign to Reduce Opioid Prescribing (CROP), entailed bimonthly, comparative, and practice-individualised feedback reports to practices, with persuasive messaging and suggested actions over 1 year. , During the intervention period, prescribing per 1,000 adults fell corresponding to 15,000 fewer patients prescribed opioids. These trends continued post-intervention, although at slower rates.
Feedback may need to be sustained for maximum effect.
There are large differences in opioid prescribing between practices, suggesting prescribing is driven by clinician habits rather than patient need.
We delivered evidence-based and theory-informed feedback reports to 316 general practices in Yorkshire, UK, every 2 months for 1 year, intended to reduce opioid prescribing by prompting physicians to think twice before starting patients on opioid medication and to review patients not currently benefiting from the medication.
15,000 fewer patients prescribed opioids at the end of the intervention year.
Prescribing of strong opioids, total opioid prescriptions, and prescribing in high-risk groups generally fell, although effects lessened after the feedback stopped.
Feedback may need to be sustained for maximum effect.
October 4, 2021 [1–5]. Prescription opioid use in the US has fallen little from 2010 peaks, despite increased awareness Despite increased awareness of the potential harms in opioid prescribing, prescription rates remain historically high in both North America and Europe [12–14]
Observed large variations in opioid prescribing, up to 10-fold in a UK study of primary care practices, suggests that physician habits and norms are a major driver, rather than patient need and evidence of benefit [.
GP prescribing ‘habits and norms’ are a key factor in the increase, rather than ‘patient need and evidence of benefit’.
Public Health England’s landmark 2019 review into prescription drug addiction concluded that one in four adults – over 11m adults in England – received a prescription for antidepressants, opioids, gabapentinoids, benzodiazepines or z-drugs in the previous year.
John Read Ph.D.
Psychiatry Through the Looking Glass
SSRIS
Tackling the Problem of Antidepressant Withdrawal
Support for people struggling to get off antidepressants is long overdue.
Posted October 10, 2021
https://www.psychologytoday.com/gb/blog/psychiatry-through-the-looking-glass/202110/tackling-the-problem-antidepressant-withdrawal
This World Mental Health Day (October 10), nearly 8 million adults in England (17 percent of the adult population) will be on prescribed antidepressants. Sadly, none of them will receive any specialised help from the National Health Service (NHS) with the withdrawal symptoms that more than half of them will encounter when they try to come off the drugs.
The number of people on antidepressants has risen every year, internationally, for 20 years. The theme for World Mental Health Day this year is “mental health in an unequal world.” In the case of psychiatric drugs, the usual problem of disadvantaged and marginalised groups not getting their fair share of resources is reversed. They are, in many cases, getting too many psychiatric drugs.
In the U.S. and UK, for example, prescription rates of antidepressants are particularly high for women, older people, and people living in deprived areas. In the part of London where I live and work, Newham, about one in every three women over 60 is on antidepressants.
These figures reflect the fact that mental health problems are, contrary to psychiatry’s dominant medical model, primarily caused by social adversities.
Until very recently, governments had the excuse of ignorance for not doing anything about the millions of people suffering withdrawal effects from psychiatric drugs. The American Psychiatric Association (APA), drug companies, and, in the UK, the Royal College of Psychiatrists and the National Institute for Health and Care Excellence (NICE), had all reported, for two decades, that withdrawal from antidepressants was rare, mild, and lasted just one or two weeks. This meant that people trying to tell their doctor about their withdrawal effects were likely to be told their original problem, e,g, depression or anxiety, was returning
With little or no help available from the NHS, or international equivalents, people have turned, in droves, to the internet for withdrawal support. There has been little research into these peer support communities. But my colleagues and I recently published a study following 13 facebook withdrawal support groups for a year. We found a total membership of 67,125, and an annual growth rate of 28 percent. Group members were 82.5 percent female, as were 80 percent of the administrators and moderators, all of whom are volunteers. The most common reason for seeking out help was failed doctor-led withdrawal.
We concluded: “Further research should focus on the methods of support and tapering protocols used in these groups to enable improved, more informed support by clinicians. Support from governments and healthcare agencies is also needed, internationally, to address this issue.”
Two years ago, I served on the expert advisory panel to a report by Public Health England (PHE), into “Dependence and Withdrawal Associated with some Prescribed Medicines.” Dr. James Davies (University of Roehampton) and I published a review of the research on antidepressant withdrawal for the PHE inquiry. We found that 56 percent of people experience withdrawal symptoms when trying to reduce or come off the drugs. Furthermore, nearly half (46 percent) of those people categorised the withdrawal symptoms as “severe,” and several studies reported that these symptoms could last for many months.
Following the PHE report, the Royal College of Psychiatrists and NICE (but not the APA or pharmaceutical companies) have updated and corrected their guidance. Finally, more people—at least in the UK—will be told about the risks of severe, protracted withdrawal before deciding whether to take the drugs, and more people will be told that they need to come off very slowly, particularly towards the very end of the tapering process.
Meanwhile, the UK’s psychotherapy, counselling, and psychology associations joined in and produced a “Guidance for Psychological Therapists: Enabling Conversations With Clients Taking or Withdrawing From Prescribed Psychiatric Drugs.” This is important because many therapists and psychologists have paid little attention to clients’ struggles with these issues, often because they see it as “the doctor’s business.”
The PHE report called for dedicated local support services for people withdrawing from prescribed medicines, and a national helpline. I am now an advisor to the “NHS England and NHS Improvement” task force commissioned to implement these recommendations. In my view, it will be medically, morally, and financially negligent for the government to continue to ignore the long-denied needs of hundreds of thousands of people across the UK, predominantly women, older people, and poorer people.
My international colleagues tell me that the UK is, on this issue at least, streets ahead of the rest of the world, which is only just beginning to acknowledged the problem exists.
World Mental Health Day seems like an opportunity to shine a light on this epidemic of unnecessary suffering and the need for those who contributed to it to step up and help fix it.