
This post written by Peter Selley and goes hand in hand with Silencing Doctors by David Healy on RxISK.org, which takes you in between the lines of what is written here and in two articles published today – a BMJ Consent Article and Vaccine contre la bronchiolite: Pfizer Essais en zone d’ombre by Ariane Denoyel for Blast, a French investigative journalism unit.
The RSV Iceberg
Recent revelations that RSV vaccine trials in pregnant women have not been totally above board will come as no surprise to seasoned followers of blog articles here . See
- Yellow and Other Virus and Vaccine Perils
- A Shot in the Dark for Pregnant People
- Women, Pregnancy, and Clinical Trials
- Another Brick in the Wall
What is so important about RSV? Not a lot.
RSV – Respiratory Syncytial Virus – is one cause of what is normally a mild lung disease – bronchiolitis. We all get infected with this bug several times during our lives. Bronchiolitis in young kids used to be regarded as trivial, especially in the better-off countries. That all changed with scaremongering tactics associated with RSV awareness. Little Belinda above has a runny nose – better rush her off to the nearest hospital with a pediatric ICU – to be on the safe side. There is no cure.
There is no cure but 99% of kids recover fine. Death is rare worldwide and vanishingly rare in developed countries.
But marketing propaganda following the recent approvals of RSV vaccines and the monoclonal antibody, Beyfortus, now called a vaccine, has led to a flood of information aimed at generating panic – among mothers in particular, See PROMISE and RESCEU
That Sinking Feeling
There is a fluffy side to this marketing.

But also a much darker side. We are now living through a marketing induced panic era. And in this case, the RSV vaccine saga demonstrates a lot that is wrong in the world of drug company clinical trials.
The main players in this world are obviously BIG Pharma who pull the strings but who could get nowhere without the puppets – a small gang of doctors, all known to each other.
Possibly most respected in this field is Prof Louis Bont. He freely admits his financial dependence on vaccine manufacturers. He heads up ReSViNET, which up until now has distanced itself from proclamations about the wonderful vaccines.
ReSViNET however also runs RSV-VW annual conferences. Most people attending don’t know that the ‘VW’ element of the title sums up their ultimate objective:- “Vaccinate the World”.
Fancy a few days in Mumbai next February for RSV-VW’24? Just don’t mention the word “preterm”.
ReSViNET is also responsible for the scariest thing after Halloween – RSV Awareness Week 2023, 6 – 10 November. Check out some ghoulish Patient Network Socials
 The other big guns (or should that be big gloves?) in RSV-land are, in no particular order: Eric Simoes, Hamish Nair and Flor Muñoz. Pfizer’s rising star Beate Kampmann appears to have recently deposed Fernando Polack, perhaps not unexpectedly.
The other big guns (or should that be big gloves?) in RSV-land are, in no particular order: Eric Simoes, Hamish Nair and Flor Muñoz. Pfizer’s rising star Beate Kampmann appears to have recently deposed Fernando Polack, perhaps not unexpectedly.
Dependence on BIG pharma is the norm – for example Dr Muñoz is a member of the data safety committee or advisory boards to Pfizer, Sanofi, AztraZeneca, GSK, Moderna, and Meissa vaccines. She receives research funding from the National Institutes of Health, the US Centers for Disease Control and Prevention, Pfizer and Gilead.
Behind the scenes are the NEJM-friendly Ghost Writers who promulgate Good News about vaccine trials.
And dare we say it behind the scenes we also find the British Medical Journal. Today’s British Medical Journal article plays straight into Pfizer’s hands – see Silencing Doctors. It manages to portray a debate as to whether volunteers in trials should be informed of possible dangers as a question as to whether it is ‘fair game‘ for women in these trials to be told:
“The risks associated with the study vaccine … may be experienced by you, but not your baby….”
The BMJ lines up two opposing teams to analyse Pfizer’s informed (sic) consent form, drafted by Advarra or whoever ran the trial, or by Pfizer – BMJ doesn’t establish this. It was approved by countless “ethical” committees or Institutional Review Boards (IRB).
A Riddle
Who said:
“Probably the most important thing for an individual to know is that, at least here in the United States, there is an independent group of folks who are overseeing research, looking out for your best interest as a patient, somebody other than the sponsor.”
Answer – Jimmy Riddle – Vice President of Research Services and Strategic Consulting at Advarra.
Advarra are probably the biggest IRB in the world. Advarra is paid a lot of money to approve clinical trial informed consent forms, quickly. The consolidation of US IRBs into 2 or 3 big businesses is not something The BMJ has covered. In this case, Advarra definitely rubber-stamped the misleading form for the RSV vaccine trial. Were they asleep at the helm?
The ultra-cautious The BMJ now must be seen to be fair – and to portray both sides of the story. From a medical point of view it looks like time for The BMJ to grow up and stop acting like a newspaper.
In 1885 the British Medical Journal felt confident to write a report on a provincial English workhouse and stated:
“The sanitary arrangements are a disgrace to the place.”
They didn’t try to find a contrarian view, or “run it past their lawyers”. Now they do and there is something badly wrong with this. The Man in the Street, in this case The Woman in the Street, knows that participants in a drug trial should, of course, be informed of possible adverse events as they evolve, and that a vaccine given in pregnancy might harm the baby.
The bizarre, misleading wording of the informed consent form – informing mothers that their baby can come to no harm as the vaccine is only given to the mother – is concerning. The fact that countless ethics committees from New Zealand to Old Zealand (Denmark) approved it shows that nobody really cares about informed consent. IRBs are looking increasingly like Spare IRBs, an afterthought, to give the industrial process some company – prevent it getting lonely.

(For more about this image google Feminist Spare Rib. We seem to have disposed of the fiery Lilith and replaced her with a docile Eve – google Lilith, Adam’s first partner).
This exposes an institutional blind spot in ethical committees and IRBs around the world. They view it as the job of the pharmaceutical company to get these statements right and assume the paperwork that comes to them is all in order. Essentially IRBs are now only responsible for ensuring the grammar is right and the layout of the form on the page looks right.
This blind spot is bigger than you might think. Woman are now being widely encouraged to get involved in drug and vaccine trials in pregnancy, and neither they, nor any IRB, appear aware that regulators have only just woken up to the fact that quite apart from ensuring companies check for teratogenicity (which they don’t always do these days), there are all sorts of other checks that should be run before any trials in pregnancy happen. See Women, Pregnancy and Clinical Trials.
The BMJ article on all this asked Pfizer some questions about the consent form and reports that “Pfizer did not answer” and that “Dr Munoz declined to comment, citing confidentiality agreements”. How much can we read into this?
Sins of Omission
The BMJ has had two articles ghostwritten for it on RSV vaccines – one in March and one now. The original material supplied by or available from the ghosts (the true authors) covers material that neither The BMJ articles mentions,
There is no reference to the massive financial support by the Gates Foundation into the quest for a RSV vaccine, which appealed to a desperate need to prevent deaths in poorer countries. Sadly subgroup analyses have shown, in South Africa, preterm birth was reported twice as often in the Pfizer RSV vaccine trial in the vaccinated group 39/469 (8.3%) compared to the placebo group 19/471 (4.0%). See FDA Clinical Review Memo.
The Odds Ratio here is 2.1576 (95% C.I. 1.2275 to 3.7926). P = 0.0075
In early 2022, GSK had terminated their RSV maternal vaccine trial for preterm birth and accompanying neonatal death on the basis of figures that were less clear cut than these. Should participants have been told about GSK’s termination?
We need to know if the pre-term births happened among White or Black South African women? Are there any implications here for White or Black American women?
GSK’s maternal vaccine is not now available anywhere. If Black South African women were primarily affected are they going to be warned about this?
Are the BMJ and everyone else keeping quiet about this because some people desperately want to overcome the reluctance of the Black community in the US to be vaccinated?
Do we know anything about the likely effects on Asian women?
Breast feeding is another omission. The benefits of breastfeeding in preventing or reducing severity of infectious disease have long been recognised but are ignored by Pfizer and the BMJ. A conservative estimate is that any benefit of RSV vaccines is similar to that of breastfeeding – although the benefits of breastfeeding are not limited to reducing RSV disease. Breast-fed babies have less to gain from this vaccination program.
Tribulations and Trials
Why did only 24 women in Australia (in 4 centres Geelong, Adelaide, Melbourne and Perth) participate? Why did Canada contribute only 55 women, and Denmark 74, while there were nearly 1000 from South Africa and 3,353 women “volunteering” in 112 sites scattered throughout the USA – including 21 sites in Texas alone.
Did it have anything to do with US “volunteers” being “compensated” by over $1000? Did marketing maneuvers like Ventavia’s suggestion that women were more likely to sign up when they were shown the ultrasound scan of their pregnancy with their partner also present make a difference? Was there any other form of persuasion? How ethical is this type of persuasion? Do IRBs check this?
Were the women told, as part of the informed consent process, that preclinical safety testing of this vaccine was limited to a few pregnant rats and rabbits? These rodents have pregnancies that last about a month.
Were they told there had been no Phase 1 studies in pregnant women?
These mothers to be were almost certainly not told that preterm birth had been added as an adverse event of special interest (AESI) as an afterthought to the Pfizer trial protocol. If any of them had asked why – would they were been told it was because GSK had terminated a trial of an almost identical vaccine early because of pre-term births?
Does Pfizer really expect people to believe that they were unaware that GSK stopped their trial because of the safety signal of preterm births and neonatal deaths?
Man Overboard
After 12 months of suspense, GSK has recently confirmed what we suspected – that Eric Simões, the lead author on the Pfizer phase 2 trial, a professor of pediatrics in Denver, Colorado – was indeed a paid member of their DSMB (Data Safety Monitoring Board) for their maternal RSV vaccine trial. This was the committee that noticed the GSK vaccine’s association with preterm births and neonatal deaths. So concerned was this committee that they recommended GSK to abandon the trial. The GSK RSVpreF3 vaccine was close enough to identical to the Pfizer RSVpreF vaccine for both companies to take legal actions against each other.
Eric, who also runs Samshoma Medical Research Inc, was, also a joint ‘author’ of the Pfizer phase 3 trial report. Eric is speaking on Video here promoting Pfizer’s wares, and, below, some of his interests.
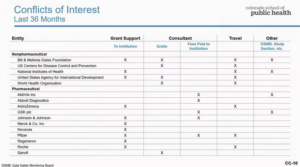
At the time pregnant moms were being recruited to Pfizer’s vaccine trial, Eric knew the reasons for the GSK trial having to stop. Are safety issues not more important than confidentiality agreements? Nancy Olivieri – the latest winner of the John Maddox Prize for Integrity in Science might have something to say about this.
Who is to blame?
The regulators fast-tracked a “priority review” of a “breakthrough therapy” vaccine for a disease that is not causing an emergency. Fast tracking suggests less thorough assessment.
They took their eyes off the ball and failed to insist on adequate preclinical testing e.g. in pregnant nonhuman primates. Whilst cursory testing for teratogenicity in rodents was done, it did not cross the regulators’ minds that these vaccines might lead to physiological changes during the mother’s pregnancy.
Synonymony
The regulators are also complicit in condoning an adverse events monitoring system that allows 5 different names for jaundice and more than 30 different terms that effectively mean “cough”. This dilutes adverse events occurrences.
Why bother with clinical trials – when regulators decide that post-marketing surveillance will compensate for a poorly-designed clinical trial they approved?
RSVP ?
Here is a list of medical colleagues I emailed to discuss safety aspects of these vaccine trials.
The only responses I received were three out-of-office automated replies (*).
- tonya.villafana@astrazeneca.com
- alejandra.gurtman@pfizer.com (x2)
- peter.marks@fda.hhs.gov
- eric.simoes@cuanschutz.edu (x2)**
- Kimberly.Center@pfizer.com
- roy.philip@hse.ie
- Beate.kampmann@lshtm.ac.uk *
- shabir.madhi@wits.ac
Emergency EMA Exit

Are there problems ahead for pharmacist and former EFPIA lobbyist, Emer Cooke, who now runs the European Medicines Agency – perhaps sending a message wearing Ukrainian colours?
She has backed Pfizer’s call for the RSVpreF vaccine to be given between 24 and 36 weeks of pregnancy. The FDA, on the other hand, acknowledging the preterm birth risks, have restricted vaccination to later in pregnancy, between 32-36 weeks.
What would you do with data like the data below?
First, note only 3 of 18 centres showed a benefit for the vaccine.
Second, the shouted from the roof-top 84% (relative) efficacy – 0.5% absolute efficacy – only seems to happen when the vaccine is given from weeks 28-32. After 32 weeks, as per FDA suggestion, the benefit plummets from 0.5% down.
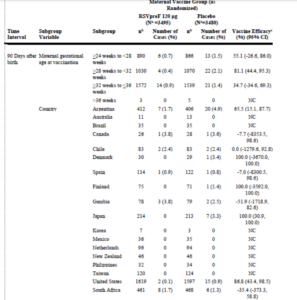
The goal of Gates Funding was to lift the burden of RSV in the developing world. Given the South African and Gambian data here, should any woman in the developing world be thinking about getting vaccinated?
One of the striking things about the BMJ article is the turn to asking bioethicists about the ethics of the consent form.
There is no addressing the issue of bioethicists, largely women, leading the stampede to get women recruited into trials during pregnancy. They may be right – the Women, Pregnancy and Clinical Trials posts argues that they are not – they are unbelievably naive – but the key point is this issue is not being addressed any more than the issue of whether we have to on pain of death refer to pregnant people rather than women. Again there may be a case for this but there are issues people are not likely to have thought about and will only come to light if there is are open discussions.
Things are no longer evolving from the bottom up. They are dictated from on high. This includes BMJ silence on some of the real issues at play in this post.
David
Translating ‘Terms’ …
So ‘the dodgy’ Poofizer vaccine, and boosters, came and went.
Pfizer shares tumbled again Monday, continuing the pharmaceutical giant’s struggles tied to declining demand for its Covid-19 products and essentially wiping out the entirety of the firm’s peak pandemic gains.
There is a lot riding on RSV for Pfizer, they need the world-backing just like they had with their triumphant acceptance of world-backing for their experimental vaccine for a novel virus.
No questions asked, forcefully jab the world, don’t even ask questions later…
Albert rubbed his hands with glee.
From the BMJ Investigation
After GSK’s trial was halted, opinion was split among clinical trial ethicists and some vaccine researchers over whether Pfizer should have informed all women participating in its trial about the potential risk or updated its consent forms. Some think that only women who had not yet been vaccinated needed to be informed, whereas others think that because there is currently neither convincing evidence nor an explanation for the increased preterm risk, informing expectant mothers would have only caused unnecessary anxiety.
Some confirmed that Pfizer continued to enrol and vaccinate women for months after the news of the potential risk of preterm birth in GSK’s vaccine trial was made public
“getting hung up on issues which are not borne out by the analysis and are distorting the benefits this vaccine can bring.”
Throw the baby out with the bathwater – slippery –
“The risks associated with the study vaccine (RSVpreF or placebo) may be experienced by you, but not your baby, since your baby will not receive the study vaccine or placebo directly.”
“However, as it has since emerged that no new participants will be included in the study, making an adjustment . . . is no longer an issue,” he said.
Brook Jackson
@IamBrookJackson
·
9h
Gonna drop this here too @TheJusticeDept
Brook Jackson
@IamBrookJackson
·
9h
Concerns over informed consent for pregnant women in Pfizer’s RSV vaccine trial https://bmj.com/content/383/bmj.p2620
Brook Jackson
@IamBrookJackson
Maybe Eric Simões who is paid by @pfizer but is also a paid member of the Data Monitoring Committee for the @GSK RSV trial recommended the trial be stopped. I suggest @FDACBER look into this…maybe even the @TheJusticeDept & @FTC
. https://davidhealy.org/yellow-and-other-virus-and-vaccine-perils/
Brook Jackson
@IamBrookJackson
·
Mar 5
During the @pfizer analyst & investor call to review RSV data held on Oct. 22, 2022, Steve Scala @TDCowen @TDCowenResearch stated that the GSK maternal RSV trial was stopped due to premature labor. How did he get this data before it was publicly available?
https://s28.q4cdn.com/781576035/file
What Pregnant People Should Know
https://www.cdc.gov/vaccines/vpd/rsv/public/pregnancy.html
Are ‘pregnant people’, ‘pregnant with anticipation’?
“Peter Selley” from the U.K.
Brook Jackson @IamBrookJackson
·
7h
IT’S SO MUCH WORSE THAN I CAN EVEN TELL YOU.
SAME FOR GSK. WE RAN THOSE TRIALS TOO.
‘Ethically Problematic’
https://childrenshealthdefense.org/defender/pregnant-women-pfizer-rsv-vaccine-preterm-birth-risk/
Before the FDA discussed the Pfizer RSV vaccine in May, “Peter Selley” from the U.K. posted a public comment to its website that said:
“It is remarkable that the FDA:
1. Do not have an obstetrician or midwife on this VRBPAC committee.
2. Allowed these trials when, it seems, there were no safety trials of this vaccine in pregnant non-human primates.
3. Allowed these large trials in pregnant women when pregnant women had been specifically excluded from smaller Phase 1 safety trials.
4. Allowed Pfizer’s phase 2 trial that involved 3-5 venous blood draws from healthy babies who would derive no benefit.
5. When notified in February 2022 by GSK of a safety signal of excess preterm births and neonatal deaths in a trial of a similar RSV vaccine, the FDA did not insist that participants in Pfizer’s Phase 3 trial should be informed of the finding.
6. Authorized an Institutional Review Board – Advarra – that approved the Phase 3 trial in pregnant women. Advarra’s informed consent form for the ongoing trial contains the advice: “The risks associated with the study vaccine (RSVpreF or placebo) may be experienced by you, but not your baby, since your baby will not receive the study vaccine or placebo directly.” (According to the trial protocol, this wording was also approved by Pfizer.)
7. Allowed the Phase 3 trial to stop early, although this may have prevented participants from experiencing adverse reactions.”
Selley appended a document detailing further safety considerations in the Pfizer trials, where he also cited Dr. Eric Simões, lead author of the Phase 2 study, who wrote in a different study, “The role of breast-feeding in preventing RSV disease and hospitalization for RSV is undisputed.”
Brook Jackson
@IamBrookJackson
·
5h
https://www.cdc.gov/media/releases/2023/p1116-rsv-doses.html
Run
The OFF…
Dr. Augusto Germán Roux reposted
Brook Jackson
@IamBrookJackson
I am suing Pfizer again.
1. Coercion of participants
2. Lack of informed consent
3. Data manipulation & fraud
4. Hidden severe adverse events (tethered spines & fetal deaths in RSV) 5. IRB fraud
Bronchiolitis: Pfizer’s cover-ups and pressure to impose a vaccine that risks the health of infants
https://www.blast-info.fr/articles/2023/vaccin-contre-la-bronchiolite-pfizer-essais-en-zones-dombre-SM_0QNVMT76A_KFpDed6BA
Another detail did not escape the vigilance of the retired doctor: “Pfizer could not have been unaware of the reasons for this abrupt termination of the GSK trial because a professor practicing in Colorado, Eric Simoes, lead author of the first article on the Pfizer trial and signatory of the second, had been a member of the Independent Safety Committee of the GSK trial.”
Asked by Blast for the purposes of this investigation and to comment on our findings, neither the EMA, nor the ANSM, nor the HAS responded to our questions within the deadline.
Also contacted, Dr. Eric Simoes did not wish to respond to Blast. His insights would undoubtedly have been invaluable, as he participated in the studies surrounding the GSK and Pfizer trials.
“the ability for Pfizer to overcome all of the obstacles during the clinical trial”
We will see.
Female-dominated praise indeed
https://youtu.be/kaFx3-abhHo
Titanic Clinical Trial Sinks
For those with an interest in the history of Pfizer’s attempts to “vaccinate the world” against RSV, here is a detailed analysis of the lethal “Lot 100” vaccine for children tested in USA in 1965.
https://undark.org/2023/10/09/rsv-vaccine-children-trials/
My Heart Will Go On
Dark
“ Even as their lives and deaths loomed over an entire field of research, Victor and Ross disappeared.”
Prince eventually shared them with Fernando Polack
Disappeared in Argentina
https://davidhealy.org/disappeared-in-argentina/
“These are really, really well-studied vaccines,” said Peter Openshaw, an RSV researcher at Imperial College London. “You couldn’t possibly launch a vaccine trial like you did back in the 1960s these days.
https://www.conservativewoman.co.uk/pfizer-presses-vaccine-on-mothers-despite-rivals-premature-birth-warning/
Really, really – they just did
“Is that possible, to have those two parallel universes?” she asked