
To the Sounds of a Drum © Nina Otulakowski November 2022
Back in the last Millenium, 1997, a book appeared called The Sovereign Individual, written by James Davidson and William Rees-Mogg (yes – the father of Jacob) about the transition to an information age. No longer would we be Numbers, we would be Free Men – See I Am Not A Number.
There was a chance for Donald Trump’s Forgotten Man to re-emerge, be seen and regain sovereignty in his or her own life. Escape the clutches of the bureaucrats and the Deep State. While this was the case, in principle, the book made it clear that we were likely heading to a new age of autocrats or oligarchs – don’t call them dictators.
A recent BBC series of podcasts called The Coming Storm, of which there are now two seasons – the first tracking the Storming of the Capitol and a second tracking what has been happening since focused on Hunter Biden’s laptop – fingers The Sovereign Individual as laying out the path from where we were in 1997 to where we are now.
A new edition of The Sovereign Individual has come out with a foreword by Peter Thiel of Paypal fame, who is a key supporter of Donald Trump. Oligarch’s Together.
Thiel has also created a company called Palantir, an operation that is eyeing NHS data and making inroads on it. This post was going to introduce readers to Palantir but Susanne, who regularly comments on posts, left a detailed comment about Palantir on the last post including the link above – given her anticipatation of this post we’ve had to do a work around.
Sovereignty

In 1649, Charles I was decapitated. He was England’s last Absolute Sovereign. He was replaced by a constitutional government. In Charles’ time the role of an Absolute Sovereign, to some extent at least, was to protect his people against the Corporations and a new merchant class then emerging. These groups fought back against his imposition of a Quarantine in the face of a plague and brought him down – as Shipwreck of the Singular outlines.
A century later, in 1848, after a series of French monarchs were decapitated or dethroned, and in the midst of a new French Revolution Marx noted that constitutional governments which had then been the handmaidens of aristocrats, and then merchants, were now switching to being the handmaidens of a middle class, the petit bourgeouis, and it was obvious given the arc of history they would inevitably become the handmaidens of the working class.
The role of governments, whether serving autocrats, merchants, or the middle class, were there to herd the people – make them conscriptable in the case of War, childbearers in the case of War, and willing to swallow the latest medicine when told to do so. See Valley of Death:
theirs is not to make reply,
theirs is not to reason why,
theirs is but to take and die
into the valley of death….
Governing the People
The collection of Data, outlined in I Am Not A Number, is all about how we are governed now. Since the end of the twentieth century government has been about governance – that is rule by numbers. Whether you blame neo-liberalism or a Deep State for ths, numbers are key.
The encroachment of numbers on the most intimate ways in which we used to think about ourselves can be seen most clearly in what once was Healthcare but is now Health Services. Getting our numbers right has replaced engaging with us to help us solve a problem getting in the way of our being able to live the life we want to live. Getting our numbers right is about getting us to live the life that Pfizer or GSK or Lilly want us to live.
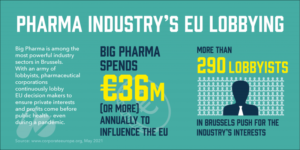
Pharma’s efforts to access our data are all about this – 36 billion Euros in lobbying European politicians annually at the last reckoning.
But as in Remind Your Sister to get Vaccinated, they also create patient groups, and support them to influence governments. Particularly targeted are patient groups that represent people with Rare Diseases – the anticipated goldmine for pharma.
There are 300 million people living with a rare disease worldwide.
Possibly the most blatant example of this subterfuge is EURORDIS – Rare Diseases Europe “We advocate for, empower and engage people living with a rare disease in Europe”.
Eurordis began well, its intentions commendable. Those who created it could never have seen that pharma would be able to transform the movement into an opportunity to deny access to clinical trial data – on the basis that people with a Rare Disease might be identified from the data.
Or that pretty quickly, pharma would make more money from Orphan Drugs for Rare Diseases than from all other drugs combined. Some of the best selling drugs for the most common conditions were brought on the market through a Rare Disease route.
Watch Dirk Vander Mijnsbrugge from Pfizer confirm the importance of a collaborative effort to address the needs of people with rare diseases at the EURORDIS’s annual European Conference on Rare Diseases and Orphan Products, recognised globally as the largest, patient-led (sic) rare disease policy event.
Read their open letter to the European Commission, reminding them of the importance:
“to the rare disease community that there is a strong European Health Data Space that upholds strong ethical principles of digital health and that includes a specific rare disease codification standard”.
Not surprisingly, conditions like DRESS and PSSD don’t seem to be invited to join the Rare Disease club.
Patient Groups as Handmaids
The euphonious Patient Partnership Index (PPI) tells us:
“First, there are the patient groups, tirelessly working to provide their communities with information, support and funds to safely get them through this difficult time. Second are the pharmaceutical companies working at pace to develop new vaccines and treatments”. The PPI is a scion of Ovid Health itself created to “bridge the divide between ‘traditional’ healthcare PR, public affairs, campaigns and patient advocacy”.
Just like the vaccine-promoting lay groups, there are hundreds of spoof organisations fuelling the fire of rare diseases on behalf of pharma.
Let’s take a look at the International Alliance of Dermatology Patient Organizations (also known as GlobalSkin), comprising over 200 patient groups. At their industry-sponsored 2021 virtual conference, the agenda included opportunities to be trained by EUPATI (the European Patients’ Academy on Therapeutic Innovation) an organisation dominated by 22 industry partners. EUPATI aims to improve health outcomes through the contribution of patients and patient representatives as valued stakeholders and to provide accessible, innovative and inclusive education that empowers patients and patient representatives
GlobalSkin has a Europe Division GlobalSkin Europe whose stated tactics include:
Using the Global Research on the Impact of Dermatological Diseases (GRIDD) project. This is the first comprehensive patient-led (sic) research on the impact of dermatological conditions in patients who live with these conditions. This impact data is vital to the success of placing dermatological disease at the top of health policy in Europe. GRIDD data unites our voices and empowers GlobalSkin-Europe to unite with a single voice and take a place at the top of health policy in Europe.
Once upon a time, language like this was harder to find but there is no effort to conceal it now. There are multiple lobbying groups with slightly different agendas. What could be more altruistic than the philanthropic non-profit organization Accumulus Synergy?
“At Accumulus Synergy, our vision is to accelerate the delivery of critical therapies to the citizens of the world. We should all want to accelerate these critical therapies to citizens globally in a safe and effective way. We use the word “citizens” intentionally, to include patients, caregivers, providers, and family members. At some point in our lives, we all benefit from medicines and vaccines.”
Surprise! – Accumulus Synergy is a consortium formed in July 2020 by 10 leading biopharmaceuticals companies: Amgen, Astellas, Bristol Myers Squibb, GSK, the Janssen Pharmaceutical Companies of Johnson & Johnson (Janssen), Lilly, Pfizer, Roche, Sanofi, and Takeda.
Patient Groups are one way into the heart of EEC policy. What else? Well Youth is the Trojan Horse of Big Pharma – see Our Unequal Struggle to get an idea of the fate of people who try to advise caution about the Horse outside the Walls.
Red Red Wine
Not UB40 but EU40. EU40 is the platform of young Pro-European Members of the European Parliament and of the 27 EU national parliaments. Their Corporate Partners?

Hold the Mayo
Its not just Pharma. According to Mayo Clinic’s marketing, brand strategy, and advertising chair Molly Biwer:
“What we’re hoping to do is gather the medical records, and it’s a very lofty goal, but the medical records of everybody in the world, so we can start to predict before diseases and conditions even happen.”
The days when doctors or anyone working in healthcare rather than health services determined priorities in the health domain are long gone.
Our desire for health – salvation – for ourselves and our children is far stronger than our desire for fast cars or gadgets. From time immemorial con artists have known how easy it is to fleece us with elixirs for love or eternal youth or sure-fire remedies to prevent the latest plague.
Sanitocracy or Sanegeddon has always been the goal toward which we have been heading.
The UK has just set up a new Health TaskForce, modelled on its Vaccine TaskForce modelled in turn on Operation Warp Speed. There could not be a better illustration of Marx’s 1848 vision of Government being the Handmaiden of whoever our current rulers might be.
The 1848 Revolutions got nowhere. The Communist party, which began in the South of France, and in 1848 had over 100,000 French members, skilled artisans mostly, with many doctors among its leaders, fell out of favor for being too pacifist. The leadership of the party decided to quit France and emigrated to the United States, settling down in Illinois, a little south of where the Mayo Clinic is now headquartered.
Footnote
With the pandemic, Blogs have been replaced by Substacks, which often note a subscription would help keep things going.
Published by Samizdat, Shipwreck of the Singular. Healthcare’s Castaways rolled out with the Vaccines. Its descriptions of a dystopian medical future and what happened the communists seem all too present now.
It is priced at $18.95, and $8.99 in kindle from each of which Samizdat gets $5, and it costs even less as El Naufragio de lo Singular.
Samizdat aims at the middle ground in health, enabling conversations to happen. A copy of any of its books for you or a friend, in lieu of an hypnotic, might help us all find a way to wake up.



The Take and the Taken…
Wales to Wisconsin…
Senator Ron Johnson, the Man for our Season
See Mary
mary H says:
November 22, 2022 at 6:28 am
Further to the above-mentioned Adam Rowland
https://davidhealy.org/august-is-the-cruellest-month/#comments
Adam Rowland Interview – Those Injured By The COVID-19 Jabs Are Being Ignored, Ridiculed & Suppressed
Joining me today on Moving Target is Adam Rowland MSc, a physiotherapist, rehabilitator, and strength & conditioning trainer for 16yrs in professional sports. Adam is here to discuss his shocking experience with the COVID-19 injections, and what he has been forced to endure simply for trying to call attention to his many severe injuries as a direct result of the jab — as confirmed by his doctor. Adam’s story is only one of millions around the world, people who are being told they’re crazy, “it’s only in your head”, or that they are merely “anxious” or “depressed” when dealing with debilitating injuries and symptoms brought on by the injections they were promised were “safe & effective”. We are here today to discuss how this is indeed happening, how their stories are being not only ignored but suppressed, but most importantly, to send out one very important message to all those feeling lost and unheard: you are not alone.
“It will make your hair drop out” – Adam
Adam was not joking
‘Processed’ – Unbelievable…
https://www.thelastamericanvagabond.com/adam-rowland-interview-those-injured-by-covid-19-jabs-are-being-ignored-ridiculed-suppressed/
Every moment in this story of Adam’s grows more incredulous as it goes on —–
https://twitter.com/oneadds
run-around, hardly cuts it —–
https://www.ronjohnson.senate.gov/2021/7/helping%20people%20be%20seen,%20heard%20and%20believed%20after%20adverse%20vaccine%20reactions
React19
@React19org
.@SenRonJohnson sees the suffering of the vaccine-injured and wants to help. On Nov. 21 he met with React19 and a large group of vaccine-injured people for an exclusive Zoom meeting to hear stories of the sick and silenced, and discuss a path forward.
https://rumble.com/v1wrlqk-senator-ron-johnson-meets-with-vaccine-injured.html
and discuss a path forward.
Britain ‘didn’t need boosters’, says top Covid tsar Sir John Bell as he claims top-up doses only prevent infections for up to 70 days
https://www.dailymail.co.uk/health/article-11485347/Britain-didnt-need-boosters-says-Covid-tsar-Sir-John-Bell.html
Britain is little better prepared for future pandemics – Former UK vaccine chief
https://www.reuters.com/world/uk/britain-is-little-better-prepared-future-pandemics-former-uk-vaccine-chief-2022-11-30/
Ex-vaccine tsar slams UK for losing Covid leadership to EU
https://www.welt.de/politik/ausland/article242399253/Kate-Bingham-Ex-vaccine-tsar-slams-UK-for-losing-Covid-leadership-to-EU.html
‘did this just happen, or is someone trying to break reality?
Annie, thank you for bringing Adam’s story up again. I shan’t repeat my previous comment to which you refer, other than to say that once these things happen to someone we know, the whole situation becomes far more real as well as more frustrating.
Anyone who isn’t aware of Adam’s story, please do follow Annie’s link above to my previous comment about him.
Any one of us, who have accepted the vaccine in good faith, could have found ourselves in the same state. Adam accepted both shots to protect others and so that he could return to his work in America. He didn’t make it back there; his health has steadily deteriorated ever since.
He fights on with every ounce of energy that he has, not only for himself but for all the vaccine injured – especially here in the UK.
The link below will show the present state of Adam – fighting for breath and fighting for tests and treatments that he needs.
https://twitter.com/Nohj_85/status/1598370715111137280?s=20&t=d9X_c1hQpvVAZw5EUHneMw
Due to the fact that the NHS seems unable to provide the tests that he needs at speed, Adam has now opened a ‘gofundme’ page in the hope that he can pay privately for the biopsies etc. that he needs. Adam’s page can be found here:- gofund.me/7839bede, should anyone feel that they are in a position to support him. There is a link, on that page, to a more detailed version of Adam’s story too.
His suffering should not have come to this – he should have been LISTENED to and BELIEVED when he first went to the variety of doctors with whom he’s had appointments. However, as we all know on here, the picture of Adam’s suffering is commonplace. It may be fairly new to us in the “vaccine” context but so common in the world of mental health and other prescribed drugs.
Adam – we shall be behind you through all your campaigning and shall look forward to celebrating with you when there is a breakthrough. I say ” when” because I know that you are not an “if” man! We feel your frustration – we share it and acknowledge that your journey is gruelling and know that you’re not about to give up any time soon.
So many people are being mugged off thinking they are doing the right thing .In a decent world they would be.
We are Parkinson’s UK
By funding the right research into the most promising treatments,
We’ll find a cure. Together.
It’s the fastest growing neurological condition in the world.
New trial to drive forward promising treatment for dyskinesia
22 November 2020
Parkinson’s UK is joining forces with US charity The Michael J. Fox Foundation and biopharma company Neurolixis to fund a £1.5m clinical trial of an exciting new drug to combat dyskinesia in people with Parkinson’s.
Home DocWire Picks
FDA Accepts New Drug Application for Parkinson’s Disease Treatment
By Team Editorial -November 11, 2022
Amneal Pharmaceuticals, Inc. (NYSE: AMRX) today announced the U.S. Food and Drug Administration (FDA) has accepted for review the New Drug Application (NDA) for IPX203 for the treatment of Parkinson’s disease
“The FDA filing acceptance of IPX203 marks another important milestone for Amneal as we strive to improve the lives and care of people living with Parkinson’s disease,” said Gustavo Pesquin, Chief Commercial Officer, Amneal Specialty. “We look forward to engaging in conversations with the FDA as we advance the application.
“Amneal aims to provide people living with Parkinson’s disease effective treatments that allow them to live their lives with less concern about their mobility and symptoms, and more freedom to choose how to spend their time,” said Pesquin.
The FDA assigned a Prescription Drug User Fee Act (PDUFA) date of June 30, 2023 to complete its evaluation of the NDA.
But This is how Amneal really treated very vulnerable people behind closed doors because they care – especially in ‘care homes’ for elders.
COVID-19 Patients Given Unproven Drug In Texas Nursing Home In ‘Disconcerting’ Move
By Vanessa Romo, NPR, April 10 2020
A bottle of hydroxycloroquine sits on a table outside the entrance to The Resort at Texas City nursing home, where Robin Armstrong, a doctor and the home’s medical director, is giving the drug to COVID-19 residents. David J. Phillip/AP
Concern is after a doctor at a Texas nursing home started giving the anti-malaria drug hydroxychloroquine to dozens of elderly patients diagnosed with COVID-19 and tracking the outcomes in what he’s calling an “observational study.”
President Trump has been an enthusiastic champion of hydroxychloroquine, calling it a “game-changer.” But some of the nation’s most respected health officials have said there is insufficient evidence showing that the 80-year-old drug, which is typically used to stave off malaria or treat lupus and rheumatoid arthritis, is a viable treatment in battling the new virus.
The Food and Drug Administration has not approved the drug for the treatment of COVID-19. The U.S. National Institutes of Health is currently tracking clinical trials of the drug.
The controversial decision to administer hydroxychloroquine at The Resort at Texas City over the last few days was made by Robin Armstrong, a physician and medical director of the nursing home.
“It’s actually going well. People are getting better,” Armstrong told NPR, adding that after just a handful of days, some of the 39 patients on the medication are showing signs of improvement.
But scientists argue that relying on observational, uncontrolled evidence can be misleading and that the only way to truly prove a drug is working is through carefully controlled clinical trials. And, contrary to Armstrong’s assertion that hydroxychloroquine “has virtually no side effects,” it is known to have serious negative health impacts. That is why so many in the medical community worry about prescribing it without such proof.
Among them is Katherine Seley-Radtke, who is a medicinal chemist at The University of Maryland, Baltimore County. She specializes in antiviral drug research, including coronaviruses.
“This is really disconcerting,” Seley-Radtke told NPR.
Armstrong admits it is difficult to quantify how much of his elderly patients’ improvement is due to the malaria drug or how they would have fared without it. Nor can he explain why other patients are not responding to the tablet doses, though he notes many are only halfway through the five-day cycle.
“To be clear, no one is worse than when they started,” he said emphatically. “From my perspective, it’s irresponsible to sit back and do nothing. The alternative would have been much much worse.”
In total, 87 people at The Resort tested positive — 56 of 135 residents as well as 31 staffers. One patient has since died.
“We know how it happened,” Armstrong said, explaining that after one staffer tested positive for COVID-19, Galveston County officials tested all other people at the facility on April 2. What they uncovered was one of the largest outbreaks in the Houston region.
“One staffer spread it to other staffers … and each of them could work with 20 to 30 patients a day,” Armstrong said.
Armstrong said he was alarmed by the test results last week and immediately began making calls to track down a source for the medicine, which is in short supply.
That’s when his political connections proved useful.
Armstrong, who is a prominent GOP activist, called Republican Lt. Gov. Dan Patrick. He says Patrick reached out to Texas state Sen. Bryan Hughes, also a Republican, who knew someone on the board of the New Jersey-based company Amneal Pharmaceuticals. The company, which makes and distributes the drug, has donated more than a million tablets nationwide, including to the states of Texas and Louisiana.
Two days later, Armstrong had received more than enough medication to begin giving it to patients. He said he started by screening those he believed would benefit most and added more people each day. He monitored their blood oxygen saturation, temperatures and how well they were breathing.
“The people who are on it were getting sicker but were not so sick that they had to go the hospital,” Armstrong explained.
He acknowledged that some families were not aware their relatives were put on the drug, saying that “for the most part,” he consulted with each nursing home resident prior to giving them on the tablets.
While the “overwhelming majority of them are awake and alert and can actually have a conversation,” Armstrong said some suffer from middle stages of dementia. In some cases, he did not discuss prescribing the tablets with anyone at all before doing so. He said it is common for physicians to prescribe new medications to patients without explicit consent from the patient or family members. “It’s not required,” he said.
He explained he was convinced by clinical studies from Europe and China showing that hydroxychloroquine helps COVID-19 patients recover from the respiratory illness because it works as “essentially an anti-inflammatory drug.”
He has some anecdotal evidence: “I’ve seen it in COVID-19 patients we’re treating” at HCA Houston Healthcare Mainland Hospital, Armstrong said.
The health care network confirmed Armstrong is a practicing physician at the hospital but would not comment on treatment of patients because of privacy concerns.
Armstrong said he is tracking the nursing home patients’ health changes daily and plans to put his findings in “some kind of report” that he hopes will add to the research on the malarial drug in relation to COVID-19.
“The problem with this is that it’s not being conducted in a proper scientific manner,” Seley-Radtke said. “It’s not being carried out with controls. It’s not being carried out under strict testing protocols and using appropriate guidelines.”
She noted warnings issued by the FDA that the drug can lead to severe problems for people with heart issues and noted that the agency urges doctors to conduct an EKG before prescribing it. (A step Armstrong said was taken on Thursday.) Another side effect involves damage to the retina.
“We know the right dosages for malaria and lupus and rheumatoid arthritis but don’t know yet what the right dosages are [for COVID-19], that’s why we are doing clinical trials to make sure we get it right,” she said.
Seley-Radtke added: “I just find it amazing that everybody, including the President, thinks that this is just no big deal to go ahead and take this.”
Armstrong denies he was swayed by politics or Trump’s championing of the malaria drug in his decision to implement it at the nursing home before it has been proven safe and effective against COVID-19.
“It’s up to a medical professional to determine how and when it would be appropriate to prescribe,” Chris Van Deusen, a spokesman for the Texas Department of State Health Services, told NPR.
Armstrong said most COVID-19-positive residents at the nursing home are asking to be on the medication “but we’re being very judicious.”
The most recent comprehensive inspection of the facility by Texas Health and Human Services occurred on July 25, 2019, according to a spokesperson.
At the time, the nursing home was cited for 14 violations of state standards. Among them, the report shows:
The facility did not properly care for residents needing special services, including: injections, colostomy, ureterostomy, ileostomy, tracheostomy care, tracheal suctioning, respiratory care, foot care and prostheses.
The facility did not store, cook and give out food in a safe and clean way.
The facility was not designed, built, equipped or well-kept to protect the health and safety of residents, workers and the public.
This side of the pond, the main contender for the EURORDIS equivalent must be the Haystack Project – “The Voice of Rare and Ultra Rare” https://haystackproject.org/
The Haystack Project is a non-profit coalition (501(c)(3) of more than 60 rare and ultra-rare
patient organizations that come together to present a coordinated, focused and unified effort to educate about the need for recognizing and addressing systemic reimbursement obstacles to patient access for rare and ultra-rare treatments. The participants are committed to working together in a collaborative and non-competitive environment, to ensure patient access to novel treatments for extremely rare conditions.
For a mere $75,000 you could become a “Sustaining Partner” in this Pharma Lobbying Organisation.
https://static1.squarespace.com/static/5966cc2220099e91326caaec/t/5e65ae2224dfaf35264127b5/1583722018967/ABOUT+HP-RCPC+and+Sponsorship+Levels-Examples.pdf
Jikky the mouse
@TheJikky
·
33m
Lol. The people who abjectly failed the British public, by subjecting them to withdrawal of standard treatment for pneumonia so that they could scare them into accepting an experimental and dangerous vaccine, gave themselves a pat on the back. They killed thousands
David Spiegelhalter
@d_spiegel
·
2h
New report from CMOs etc on Covid https://gov.uk/government/publications/technical-report-on-the-covid-19-pandemic-in-the-uk… Chapter 11 ‘Communications’ says sensible things. “The media medics, and most specialist health and science correspondents, provided challenge, were well informed and generally relayed accurate technical messages clearly”
Thread
https://twitter.com/d_spiegel/status/1598250330176749569
Jikky the mouse
@TheJikky
·
18m
Replying to
@TheJikky
@profnfenton @joshg99 @ClareCraigPath @jengleruk @MartinNeil9 @RuminatorDan
Independent Report
https://www.gov.uk/government/publications/technical-report-on-the-covid-19-pandemic-in-the-uk
Independent Report – Authors
https://www.gov.uk/government/publications/technical-report-on-the-covid-19-pandemic-in-the-uk/list-of-chapters-authors-reviewers-and-contributors
Note 2: many authors, reviewers and contributors have changed organisations during the period 2020 to 2022
That, has to be, The ‘Icing on the Cake’…
In the Hall of the Mountain Kings…
Juan Gérvas
@JuanGrvas
·
1h
Dos muertes por lecanemab en el ensayo clínico. Alzheimer. Two deaths. This clinical trial death is the second thought to be associated with the antibody called lecanemab.
Second death linked to potential antibody “It was a one-two punch,” Castellani says. “There’s zero doubt in my mind that this is a treatment-caused illness and death. If the patient hadn’t been on lecanemab she would be alive today.”
https://www.science.org/content/article/second-death-linked-potential-antibody-treatment-alzheimer-s-disease
A 65-year-old woman who was receiving a promising experimental treatment to slow the cognitive decline caused by her early Alzheimer’s disease recently died from a massive brain hemorrhage that some researchers link to the drug. The clinical trial death, described in an unpublished case report Science has obtained, is the second thought to be associated with the antibody called lecanemab. The newly disclosed fatality intensifies questions about its safety and how widely lecanemab should be prescribed if ultimately approved by regulators.
Chris Whitty and Patrick Vallance Warn Britain faces “Prolonged Period” of Excess Deaths – Not Due to Covid, But Due to the Lockdowns
Britain will face a “prolonged period” of deaths due to the pandemic — but not from the Covid virus itself, Sir Chris Whitty and Sir Patrick Vallance said today, but from the (entirely foreseeable) collateral damage done by the measures taken to ‘contain’ it. MailOnline has more.
https://dailysceptic.org/2022/12/01/chris-whitty-and-patrick-vallance-warn-britain-faces-prolonged-period-of-excess-deaths-not-due-to-covid-but-due-to-the-lockdowns/
More – The Narrative Control
https://www.dailymail.co.uk/health/article-11491871/Chris-Whitty-warns-Britain-faces-prolonged-period-excess-deaths.html
Robert F. Kennedy Jr
@RobertKennedyJr
·
13h
Please get our new book as a holiday gift for sleeping relatives. It’s reveille time! Wake up, everybody!
https://www.amazon.com/Cause-Epidemic-Sudden-Childrens-Defense/dp/1510776397
Robert F. Kennedy Jr
@RobertKennedyJr
Dr. Eli David
@DrEliDavid
Covid vaccine effectiveness countdown
Sound On
https://twitter.com/DrEliDavid/status/1597841658204213248
In the Hall of the Mountain King
from ‘Peer Gynt’ …
Moonshot…
Sir Patrick Vallance
@uksciencechief
·
Nov 30
Developing and deploying safe, effective diagnostics, therapeutics, and vaccines within the first 100 days of a pandemic can save countless lives. The 100 Days Mission is ambitious, but achievable and essential.
CEPI
@CEPIvaccines
·
Nov 30
What will it take? Read our “What Will it Take” report, which outlines 5 areas of innovation needed to make delivery of pandemic vaccines within 100 days a reality…
https://bit.ly/3ESprgm
Show this thread
https://twitter.com/CEPIvaccines/status/1597897453235159040
https://cepi.net/wp-content/uploads/2022/11/CEPI-100-Days-Report-Digital-Version_29-11-22.pdf?swcfpc=1
Call to action Stopping or preventing the next pandemic, let alone in 100 days, is not something a single country or organisation can do alone. Nor will it likely be achieved by simply funding vaccine developers and biotech companies to advance innovative work. Success will require advancements in organisation, governance, and financing of global preparedness systems, and multiple, interconnected scientifically guided collaborative efforts.
The ‘moonshot’ goal of making a vaccine against a new pandemic pathogen in 100 days is ambitious, but this research exercise shows it is not impossible. Several organisations, including CEPI have laid out ambitious programmes leveraging many of the approaches described in this paper. Other countries and regions have begun additional activities such as expanding vaccine manufacturing so that ready, prepositioned manufacturing capacity is less likely to be a limiting step in responding to the next pandemic.
2013-06-14 — The RIAT team sends an email to GSK, Sir Andrew Witty (CEO) and Patrick Vallance (President of Pharmaceutical R&D), notifying them of the RIAT article publication and requesting that if they plan to restore any old GSK trials, they respond as soon as possible.
https://study329.org/correspondence-with-gsk/
‘Moonshot goal’
https://www.amazon.co.uk/Moonshot-Pfizers-Nine-Month-Impossible-Possible/dp/B09R4RNJG3/ref=sr_1_1?adgrpid=1185274457991548&hvadid=74079861286355&hvbmt=be&hvdev=c&hvlocphy=133614&hvnetw=o&hvqmt=e&hvtargid=kwd-74079813940725%3Aloc-188&hydadcr=11889_1899855&keywords=moonshot+albert+bourla&qid=1670090713&sr=8-1
What will it take?
Witty and Vallance causing a RIAT…
Link the Links…
“Found dead at home”
“Not a spurious causation” John Campbell
‘Died Suddenly’ is hitting the rounds.
A knock on my van-door, and a neighbour came in who was very distressed. Her best friend, who I have met, and I found to be lively, funny, witty and warm was found dead in her bed a few week-ends ago.
The neighbour was upset because she couldn’t believe how she had died; they had agreed to meet for coffee the morning before the death and she didn’t turn up. Apparently, the doctor put COPD on the death certificate but my neighbour said she occasionally used an inhaler and that was it.
I did ask when was her booster and it was 3-4 weeks previously. The family didn’t have a post-mortem as they didn’t want to cause a fuss.
“Found dead at home”
“Not a spurious causation” John Campbell
Dr Clare Craig (not one of her impersonators)
@ClareCraigPath
·
Dec 3
35 autopsies reviewed within 20 days of Vx.
Myocarditis seen in 5 of them who died within a week
No similar findings in historical matched controls.
Right side of heart worse affected as if it could be direct damage from LNPs / mRNA draining to heart.
https://link.springer.com/article/10.1007/s00392-022-02129-5#Sec3
John campbell
@Johnincarlisle
German photographs https://youtu.be/j_DdSMn55cA via
@YouTube
These are the German photographs, where are the UK and US and Australian and NZ photographs?
https://www.youtube.com/watch?v=j_DdSMn55cA
‘High Index’ of suspicion –
The shabby dishonesty of Matt Hancock’s ‘diaries’
https://www.spectator.co.uk/article/the-shabby-dishonesty-of-matt-hancocks-diaries/
‘Standing in my kitchen in Suffolk after a quiet New Year’s Eve, I scanned my newspaper for clues as to what might be lurking around the corner.’
So run the opening words of yesterday’s first extract of Matt Hancock’s Pandemic Diaries: The Inside Story of Britain’s Battle Against Covid. 1 January. New Year’s Day. And our hero – modest, unassuming, but eternally vigilant, eyes always scanning the horizon – is on duty, even when most of us are nursing a foggy head.
Of course, we know now what this man of destiny didn’t know then: that the ‘news-in-brief story about a mystery pneumonia outbreak in China’ that catches his eye is the harbinger of the vast global story that is to change all of our lives. Yet here he was, if we’re to believe this, that very day: noticing the tiny news item that turned out to be a message from his bleak and momentous future.
But, of course, he didn’t. And it’s a mild insult to all of our intelligence to be asked to imagine that he did.
Who keeps a note of a news-in-brief item, unless it’s an especially rude or funny one? Also, nobody in any diary in the history of diaries has written a phrase like ‘standing in my kitchen in Suffolk after a quiet New Year’s Eve’.
So Matt Hancock’s book may be any number of things – a grotesque cash-in, a litany of self-justification and special pleading, a valuable insider account, a triumphant vindication of a much-maligned man doing his best in difficult circumstances, or a muddled mixture of all these things.
Take your pick: most people already will have. Firm opinions will have been formed on the matter long before anybody has had the chance to read the extract, let alone had the pleasant surprise of finding Mr Hancock’s book in their Christmas stocking.
The one thing that this book is not, though, is what the title proclaims it. It is, instead, an example of a particularly shabby new non-fictional form: the ‘diary’ written in retrospect. Even by the admission of his publisher, the book that Mr Hancock has cooked up with the political journalist Isabel Oakeshott has ‘(drawn) on a wealth of never-before-seen material, including official records, his notes at the time and communications with all the key players in Britain’s Covid-19 story’ – which is to say it’s not a diary at all. It’s a memoir in diary form.
Why does that matter? Because presenting the story as a diary is a literary device – and, especially in the case of a political memoir, it’s a rhetorical one. It affects to give the document the immediacy and authenticity of a real-time account rather than a hazy and self-serving after-the-event write up, with all the hindsight that brings.
It is a fundamentally dishonest and trivial way to present a serious account of events. That’s okay for ‘autofiction’, which is a literary rather than a historical form. It’s not appropriate for something that’s purporting to be a contribution to historical truth. The model for this ersatz form, I suspect, is not from politics but showbiz: Piers Morgan’s very enjoyable, gossipy, successful and entirely unreliable ‘diaries’, cobbled together long after the event, of his time as a newspaper editor and later telly presenter.
Diaries – real diaries, kept contemporaneously and published as close to unexpurgated as is possible within decency and the law – are a historical document past price. Pepys is Pepys because when he recorded the great fire of London, he didn’t know he was recording the Great Fire of London. And his personality – vivacious, lecherous, chaotic, priggish here and companionable there – comes through as it was, not as he wished it to be.
Kenneth Williams, Joe Orton, even the anonymous author of the logorrheic diaries found in a skip and edited by my old friend Alexander Masters as A Life Discarded: the quick of life is in these things. Social historians of David Kynaston’s stripe can draw riches from the mundane diaries of ordinary people living through the era under examination. Some of the pungent ephemera of life is only really preserved in the diaries of the people who cared about, or just noticed, that ephemera while it was happening.
And the great political diaries – think ‘Chips’ Channon, Tony Benn, Alan Clark, Alastair Campbell or Chris Mullin – offer an insight into politics and a flavour of the writer’s personality that no other form can. But their value is exactly that they are written while the events that they describe take place. They show their writers changing their minds, being surprised by developments, getting it wrong. They do not flatter their authors’ vanity (or the worthwhile ones do not; Tony Benn’s diaries, for instance, live in my heart for the guileless way he describes reaching into his pocket and popping what he thinks is a Polo mint into his mouth, only to find out it’s a mothball). They certainly do not contain clumsy pseudo-literary prolepsis of the absurd ‘this news-in-brief item caught my eye’ type.
Yet by associating himself with these predecessors, Hancock bids to draw subliminally on their prestige and hopes, perhaps, to share in the affection we often find ourselves feeling for the authors of real-time diaries. Showing, as he’d see it, ‘the guy behind the podium’. Well. That affection comes from how unguarded and uncalculating such documents are: we trust the authors to be honest because, in writing from the passing passions of the moment, they can’t but reveal themselves in all their flawed humanity.
Putting in a handful of carefully chosen flaws retrospectively just won’t cut it. And as far as human feeling goes, Hancock offers only notes of frustration – he being the only person who properly appreciates the urgency of the crisis, obviously – when he is thwarted. He registers this more than once with the sublimely Partridgean one-word payoff: ‘Infuriating!’
So I don’t purport to judge the truth or otherwise of the content of Hancock’s account. That will be sifted by proper historians, cross-checked with the accounts of other participants in events, combed through by the various Covid postmortems both official and unofficial.
My complaint, here, is about the form. It’s a memoir, and it should be honest about that. These resemble the diaries of a Chips Channon or an Alan Clark as Swarovski crystals resemble diamonds.
‘died suddenly’ …
But the MHRA is an internationally respected independent watchdog and for now those about to receive the first jabs will rely on its ruling.
Giving the analogy of climbing a mountain, she said: “If you’re climbing a mountain, you prepare and prepare. We started that in June. By the time the interim results became available on 10 November we were at base camp.
“And then when we got the final analysis we were ready for that last sprint that takes us to today.”
https://www.bbc.co.uk/news/health-55145696
Britain’s health regulator approves Pfizer/BioNTech COVID vaccine for infants
Story by Reuters • 1h ago
https://www.msn.com/en-gb/health/other/britain-s-health-regulator-approves-pfizer-biontech-covid-vaccine-for-infants/ar-AA14XLza?ocid=msedgdhp&pc=U531&cvid=8ab5a9d490cb41c08e4410572efd88c1
LONDON (Reuters) – Britain’s health regulator on Tuesday authorised the use of a version of a COVID-19 vaccine made by Pfizer and BioNTech in infants and children aged 6 months to 4 years.
Whether the vaccine is eventually deployed in this age group depends on a recommendation from the UK’s Joint Committee on Vaccination and Immunisation (JCVI), which advises on which shots are used as part of the national vaccination programme.
The endorsement from the Medicines and Healthcare products Regulatory Agency (MHRA) is based on data from an ongoing clinical trial involving 4,526 participants.
BioNTech SE
@BioNTech_Group
·
21h
We announced today with @Pfizer the submission of an application to the @US_FDA
for the emergency use authorization (EUA) of the Omicron BA.4/BA.5-adapted bivalent #COVID19 vaccine for children 6 months through 4 years of age.
https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-submit-application-us-fda-emergency-use-1
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received COMIRNATY® (COVID-19 vaccine, mRNA) or Pfizer-BioNTech COVID-19 Vaccine. The observed risk is higher among adolescent males and adult males under 40 years of age than among females and older males, and the observed risk is highest in males 12 through 17 years of age. In most of these people, symptoms began within a few days following receipt of the second dose of vaccine. The chance of having this occur is very low
https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-submit-application-us-fda-emergency-use-1
Pfizer Disclosure Notice
The information contained in this release is as of December 5, 2022. Pfizer assumes no obligation to update forward-looking statements contained in this release as the result of new information or future events or developments.
This release contains forward-looking information about Pfizer’s efforts to combat COVID-19, the collaboration between BioNTech and Pfizer to develop a COVID-19 vaccine, the BNT162b2 mRNA vaccine program, and the Pfizer-BioNTech COVID-19 Vaccine, also known as COMIRNATY (COVID-19 Vaccine, mRNA) (BNT162b2) (including the Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), including a submission to the U.S. Food and Drug Administration (FDA) for an Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine as the third 3-µg dose in the three-dose primary series for children 6 months through 4 years of age, planned regulatory submissions, qualitative assessments of available data, potential benefits, expectations for clinical trials, potential regulatory submissions, the anticipated timing of data readouts, regulatory submissions, regulatory approvals or authorizations and anticipated manufacturing, distribution and supply) involving substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. Risks and uncertainties include, among other things, the uncertainties inherent in research and development, including the ability to meet anticipated clinical endpoints, commencement and/or completion dates for clinical trials, regulatory submission dates, regulatory approval dates and/or launch dates, as well as risks associated with preclinical and clinical data (including Phase 1/2/3 or Phase 4 data), including the data discussed in this release for BNT162b2, any monovalent, bivalent or variant-adapted vaccine candidates or any other vaccine candidate in the BNT162 program in any of our studies in pediatrics, adolescents, or adults or real world evidence, including the possibility of unfavorable new preclinical, clinical or safety data and further analyses of existing preclinical, clinical or safety data, including the risk that additional data against newer Omicron sublineages could differ from the data discussed in this release; the ability to produce comparable clinical or other results, including the rate of vaccine effectiveness and safety and tolerability profile observed to date, in additional analyses of the Phase 3 trial and additional studies, in real world data studies or in larger, more diverse populations following commercialization; the ability of BNT162b2, any monovalent, bivalent or variant-adapted vaccine candidates or any future vaccine to prevent COVID-19 caused by emerging virus variants; the risk that more widespread use of the vaccine will lead to new information about efficacy, safety, or other developments, including the risk of additional adverse reactions, some of which may be serious; the risk that preclinical and clinical trial data are subject to differing interpretations and assessments, including during the peer review/publication process, in the scientific community generally, and by regulatory authorities; whether and when additional data from the BNT162 mRNA vaccine program will be published in scientific journal publications and, if so, when and with what modifications and interpretations; whether regulatory authorities will be satisfied with the design of and results from these and any future preclinical and clinical studies; whether and when submissions to request emergency use or conditional marketing authorizations for BNT162b2 in additional populations, for a potential booster dose for BNT162b2, any monovalent or bivalent vaccine candidates or any potential future vaccines (including potential future annual boosters or re-vaccinations), and/or other biologics license and/or emergency use authorization applications or amendments to any such applications may be filed in particular jurisdictions for BNT162b2, any monovalent or bivalent vaccine candidates or any other potential vaccines that may arise from the BNT162 program, including a potential variant-based, higher dose, or bivalent vaccine, and if obtained, whether or when such emergency use authorizations or licenses will expire or terminate; whether and when any applications that may be pending or filed for BNT162b2 (including any requested amendments to the emergency use or conditional marketing authorizations), any monovalent or bivalent vaccine candidates (including the submission to the FDA for an Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine), or other vaccines that may result from the BNT162 program may be approved by particular regulatory authorities, which will depend on myriad factors, including making a determination as to whether the vaccine’s benefits outweigh its known risks and determination of the vaccine’s efficacy and, if approved, whether it will be commercially successful; decisions by regulatory authorities impacting labeling or marketing, manufacturing processes, safety and/or other matters that could affect the availability or commercial potential of a vaccine, including development of products or therapies by other companies; disruptions in the relationships between us and our collaboration partners, clinical trial sites or third-party suppliers; the risk that demand for any products may be reduced or no longer exist which may lead to reduced revenues or excess inventory; risks related to the availability of raw materials to manufacture a vaccine; challenges related to our vaccine’s formulation, dosing schedule and attendant storage, distribution and administration requirements, including risks related to storage and handling after delivery by Pfizer; the risk that we may not be able to successfully develop other vaccine formulations, booster doses or potential future annual boosters or re-vaccinations or new variant-based or next generation vaccines; the risk that we may not be able to maintain or scale up manufacturing capacity on a timely basis or maintain access to logistics or supply channels commensurate with global demand for our vaccines, which would negatively impact our ability to supply the estimated numbers of doses of our vaccines within the projected time periods; whether and when additional supply agreements will be reached; uncertainties regarding the ability to obtain recommendations from vaccine advisory or technical committees and other public health authorities and uncertainties regarding the commercial impact of any such recommendations; challenges related to public vaccine confidence or awareness; uncertainties regarding the impact of COVID-19 on Pfizer’s business, operations and financial results; and competitive developments.
A further description of risks and uncertainties can be found in Pfizer’s Annual Report on Form 10-K for the fiscal year ended December 31, 2021 and in its subsequent reports on Form 10-Q, including in the sections thereof captioned “Risk Factors” and “Forward-Looking Information and Factors That May Affect Future Results”, as well as in its subsequent reports on Form 8-K, all of which are filed with the U.S. Securities and Exchange Commission and available at www.sec.gov and www.pfizer.com.
Dr Clare Craig (not one of her impersonators)
@ClareCraigPath
·
32m
You saw the FDA approve for 6 months and up on the basis of utterly shoddy data.
You might have said that would never happen in the UK.
You drew a red line that babies should not be used as a human shield in this way.
Your red line has been crossed.
Dr Clare Craig (not one of her impersonators)
@ClareCraigPath
·
2h
6 month to 4 year olds!
Pfizer/BioNTech “has met the MHRA’s required safety, quality and effectiveness standards” – what standards?
https://gov.uk/government/news/pfizerbiontech-covid-19-vaccine-authorised-for-use-in-infants-and-children-aged-6-months-to-4-years…
The video below described the trial which the MHRA “carefully reviewed data from.”
HART
@hartgroup_org
1/ Dr Clare Craig explains why the FDA should NOT have granted approval for roll-out in the 6 month to 4 yr old children cohort
This trial should have been deemed null and void.
The regulators are failing to do their job.
Share widely & follow @hartgroup_org for updates
https://twitter.com/hartgroup_org/status/1537458392414969856
“twist the data”
UK approves Covid vaccines for BABIES
https://www.msn.com/en-gb/health/other/uk-approves-covid-vaccines-for-babies/ar-AA14YlB2
Covid vaccines were today approved for babies in Britain, in a move likely to spark huge controversy.
Regulators gave the green light for all infants older than six months to get a special, low-dose formulation of Pfizer’s jab.
Its decision opens the door for No10’s vaccine advisers to choose whether under-5s should be jabbed as part of the UK’s vaccination strategy.
Authorities have so far held out on recommending jabs for infants due to concerns that the benefits don’t outweigh any potential risks. Children rarely get seriously ill with the coronavirus, and the majority are thought to have already been infected.
“We started that in June” – June…
Waste Not Want Not – Want More
PHARMA
COVID-19 tracker: Pfizer eyes up to $15B in annual mRNA shot sales by 2030
By Zoey Becker, Kevin Dunleavy, Fraiser Kansteiner, Angus Liu
Dec 13, 2022 10:05am
ModernaPfizerCOVID-19Paxlovid
COVID-19 vaccine
As the COVID-19 pandemic continues to unfold, we’re tracking the biopharma industry’s response here.
Pfizer is confident it can gin up megablockbuster sales in mRNA beyond its COVID-19 vaccine.
Please read below for the latest updates. Daily COVID-19 tracker entries from April 14 to Aug. 31 can be found here.
UPDATED: Tuesday, Dec. 13 at 10:05 a.m. ET
Lower COVID revenues? No problem. That’s the word according to Pfizer executives, who said at an investor event Monday that annual sales of the company’s mRNA vaccines could reach $10 billion to $15 billion by 2030. Pfizer’s big bet on its non-COVID-19 mRNA projects comes as coronavirus-related sales are expected to plunge over the next few years, Reuters reports. Pfizer didn’t break down specifical sales projections for each of its mRNA contenders, though the company noted its respiratory syncytial virus (RSV) shot, pegged for launch in 2023, could peak in 2027 with revenue above $2 billion.