
Editorial Note: This post is by Johanna Ryan. As with all posts by Jo, it unearths angles on current stories that everyone else seems to have missed.
A column here last month followed the legacy of Study 329 into the present. By taking apart one 2015 study of Vraylar, a new antipsychotic, I tried to show that clinical research in 2015 is even more ghost-written, and more tightly controlled by industry, than that infamous 1990’s study. I had planned to follow up with a look at what the official authors and contract researchers involved in that Vraylar study were up to now.
Then Robert Califf was nominated to head the U.S. Food and Drug Administration, and I recognized the real Ghost of Research Future. He was right there on Capitol Hill in a white coat and a winning smile, holding forth to politicians and the media at his Senate confirmation hearing.
Two facts about Robert Califf are beyond question. He is an expert on clinical trials, who is already seen as a leading architect of the future of medical research. And as the New York Times put it, he has “deeper ties to the pharmaceutical industry than any FDA commissioner in recent memory”. A lot of senior figures in medicine support Califf in spite of his ties to Pharma. The guy is just so bright, and understands the nuts and bolts of drug research so well! Surely a person like this is more useful than some outsider who offers only a squeaky-clean resume, they argue.
Other researchers and healthcare activists are not so sure. They point to clinical trials run by Califf and his Duke colleagues for Xarelto, a new anti-clotting drug, that were flagged by FDA reviewers as being biased in Xarelto’s favor. The studies were funded by Johnson & Johnson, and the concerns were serious enough that two senior reviewers voted against Xarelto’s approval. Former FDA cardiovascular analyst Thomas Marciniak called Califf “one of the architects” of a broken clinical trial system run by drug companies, a feat for which he should be “held accountable, not appointed to run the FDA.”
Most of the senators at his confirmation hearing shared a rare bipartisan enthusiasm for Califf, but a few had their doubts. Vermont’s independent senator Bernie Sanders, currently running for president as a Democrat, wants the FDA to take on prescription drug price-gouging. He doubts Califf is the man for the job. Senator Elizabeth Warren thinks the FDA must insure new blockbuster drugs like Xarelto are actually effective and safe. She has asked Califf for a look at the terms of his contracts with the drug companies that funded his research.
The Duke Clinical Research Institute
The Duke Clinical Research Institute (DCRI) will likely be Senator Warren’s first stop. Califf cofounded the DCRI and was its first director. Since then he’s helped launch a dizzying array of Institutes, think tanks and public-private partnerships: the Clinical Trials Transformation Initiative, the Clinical Research Forum, the Duke Translational Medicine Institute, which he headed just prior to joining the FDA, and more. But the DCRI is his signature achievement, his baby, so to speak. And if Duke University was the mother and Califf the midwife, the father was Research Triangle Park, the Silicon Valley of corporate medical R&D just down the road from Duke.
Back in the 1990’s, the problem posed by Study 329 was medical school researchers letting their work be funded and co-opted by industry. By 2015, things were worse. It was common to see these same researchers signing on to clinical trials designed and controlled by industry from the start, with minimal input from their universities. The Vraylar studies were an example of med school faculty lending their prestige and credibility to work that was actually done in dozens of commercial Contract Research Organizations (CROs) far from the campus, and written up by medical communications agencies hired by the drugmakers.
Robert Califf’s DCRI is a new hybrid: a CRO built right into the university, but whose mission is to solve whatever research questions matter to its paying customers, like any other CRO. DCRI describes itself as:
“… the only one of its kind that can offer all the services of a commercial contract research organization (CRO) with the academic credibility and expertise of an academic research institute. From planning to execution to publishing results, the DCRI excels at every facet required for a speedy, effective research project. Our unique operational model ensures that all aspects of a project account for both our dedication to patients and the business needs of our sponsors.”
Some of this research is done at Duke. Most, however, takes place at outside research sites recruited and managed by the trial experts at DCRI. In cardiology, DCRI touts its ability to run global commercial megatrials in dozens of countries. [https://www.dcri.org/our-research/cardiology/Megatrials]
DCRI also provides “strategic communications”: everything from crafting effective journal articles and conference presentations, to designing CME programs that make sure prescribing doctors absorb the key results of the sponsors’ research. It connects companies with “thought leaders” who “are uniquely positioned to understand the operational, financial, and regulatory implications of numerous project designs, to the great benefit of our clients.” [https://www.dcri.org/our-services/thought-leadership]. It even offers clinical-research classes delivered directly to corporations through DCRI Learn: “Let DCRI customize an experienced-based learning program for your employees. Proposals are confidential and programs individualized.” [https://dcri.org/learn]
Faculty Connection, LLC
It’s hard to imagine an industry-sponsored project Duke faculty members could sign up for that would not “fit within the mission of the university”. [https://www.youtube.com/watch?v=XLG6FLvw18Q]. Yet in 2005 “Faculty Connection, LLC” was formed to help professors do just that, with Robert Califf on its board of directors. FC negotiates contracts (and aims to limit confidentiality clauses to “five years or less”), takes care of required conflict-of-interest reports, and “shields consultants from personal liability” through its LLC structure, as this 2011 video explains. [https://www.youtube.com/watch?v=nF2DbKxnT6A]. In return it charges a 25% overhead fee which it says is often paid by industry; if not, it takes 20% of the faculty member’s earnings.
Most of Califf’s personal consulting fees for work with industry appear to have come through FC. If so, the “nonprofit” to which he famously donated his fees would usually have been DCRI itself, through its fellowship programs for promising young Duke researchers.
The Xarelto story: Safe and effective compared to what?
“Bias in choosing the question is a much bigger problem than lying about the data.”
– Robert Califf, February 12, 2013
Xarelto is one of several new anti-coagulant drugs approved in recent years to prevent strokes and other clot-related complications, especially in people with heart-rhythm disorders such as atrial fibrillation. All aim to replace a much older, cheaper and more well-known drug called warfarin.
Anti-coagulation or “blood thinning” is a trick that needs to be done just right. It must work well enough to prevent formation of clots that can lead to strokes or heart attacks. But if it works too well, even minor internal bleeding can become life-threatening. That’s why warfarin requires careful dosing, limiting certain foods and alcohol, and periodic tests to make sure your blood level is in a therapeutic range. It’s also why people with milder cardiac conditions are often advised to take aspirin instead, which is less effective but simpler to use.
What drugs like Xarelto and Pradaxa promised was anti-clotting action as good as or better than warfarin, but in a convenient once-daily dose with no need for monitoring. That was Dr. Califf’s message when he presented the ROCKET-AF trial to the American Heart Association in 2011. Even then, he faced pointed questions about conduct of the trial, especially in the non-US trial centers. Warfarin doses for subjects in the control group were so poorly controlled that their blood levels were often outside therapeutic range. That set them up for a higher risk of strokes – and also made Xarelto look better than it was.
Cardiology expert Steven Nissan considered it a “fatal flaw” – and added that those more cynical than he might think the trial committee had planned it that way. The FDA reviewers agreed, pointing out that warfarin treatment in the ROCKET-AF trial was worse than either the Pradaxa trials or older studies. Patients in the U.S. who had access to good quality warfarin treatment might actually be in more danger, not less, on Xarelto.
ROCKET-AF had been billed by Califf and Duke as more convincing because it was a “real-world” trial, in which warfarin doses would be left to clinicians’ judgment rather than subject to a strict protocol. This could also lead to trouble if local trial sites had an incentive for sloppy use of warfarin, or trial designers had an incentive to choose second-rate clinicians. Xarelto would be “safe and effective” – but compared to what? Bias in choosing the question would have the same effect as rigging.
The reviewers’ next worry was a problem Xarelto shared with Pradaxa: the convenience of once-daily dosing with no blood tests, which just might be too loose to guarantee safety. Prior to the trial, the FDA had recommended that Xarelto be tested in a twice daily dose, due to its short half-life, but the ROCKET-AF group used the once-daily dose anyway – just like the trials of competitor Pradaxa. Critics charged that the makers of both drugs had sacrificed safety for the marketing value of “convenience” as an incentive to put more patients on the expensive new products.
There were two more potential safety problems: Stopping Xarelto had led to 22 strokes in 30 days, compared to just six in the control group – even though the Xarelto group were transitioned onto warfarin. It seemed quite possible that stopping Xarelto put patients in a “prothrombic state”. This could leave them in a dangerous bind if they needed to stop Xarelto, in order to have surgery for example.
Finally, unlike warfarin, whose effects can be reversed by vitamin K or plasma, there was no antidote for drugs like Xarelto and Pradaxa. In an emergency, patients could bleed uncontrollably and doctors might be unable to stop it.
The Portola story: Who needs an antidote?
To your family doctor, the lack of an antidote might sound scary – as it did to the FDA reviewers. However, developers of Xarelto insisted there was no cause for concern. A 2014 paper co-authored by Robert Califf pointed out that “all-cause death after bleeding was similar in the rivaroxaban arm compared to warfarin” in their study. It also noted that “although physicians may express a desire for specific ‘reversal agents’, these interventions are not used in the vast majority of bleed events,” and the reversal agents for warfarin were “sub-optimal” anyway. Reading that, physicians might think their worries were overblown after all.
So they might have been surprised to hear that DCRI was already working on an antidote to both Xarelto and Eliquis, made by a company called Portola Pharmaceuticals – with Robert Califf on the board of directors. In 2014 Portola announced that FDA had granted Fast-Track status to its antidote, andexanet-alfa, opening the way for faster approval with less evidence. Fast Track is reserved for drugs that address a life-threatening condition for which no approved treatments exist – and uncontrolled bleeding in Xarelto or Eliquis users qualified.
It wasn’t until January 26, 2015, when Califf joined the FDA as interim deputy commissioner, that he announced he would be retiring from Portola’s board and selling his shares. Fortunately this was after Portola’s January 9 announcement of the success of its Phase III trials, which sent its stock price soaring. (Califf also sold off a “significant” investment in N30 Pharma, a startup developing drugs for cystic fibrosis.) Duke scientists continue to work with Portola on another anticoagulant called betrixaban, and with Perosphere Pharma on PER977, an antidote to edoxaban which may reverse other anticoagulants as well. (Meanwhile, Boehringer-Ingelheim gained fast-tracked FDA approval for Praxbind, an antidote for the “life-threatening” effects of its own drug, Pradaxa. A single Praxbind injection will cost somewhere in excess of $3,000 wholesale.)
Regado
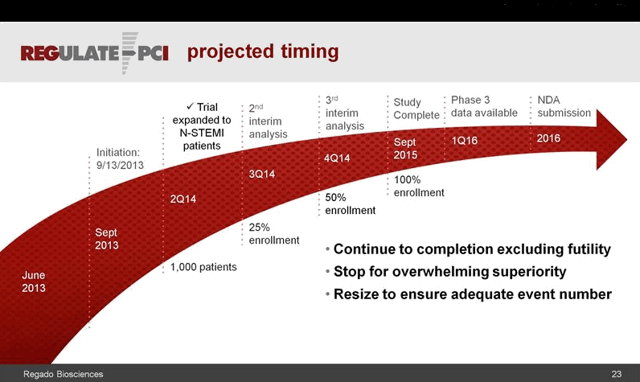
This wasn’t Califf’s only foray into anticoagulants and antidotes, or the business end of drug development. In March 2014 a similar fanfare greeted the announcement of fast-track status for Regado Bioscience’s anti-clotting drug and antidote combination, dubbed REG1 and REG2, for use in stenting and other cardiac procedures. A small number of serious allergic reactions in the Phase 2 trial three years earlier were not enough to slow the progress of Regado’s potentially life-saving therapy. [http://www.tctmd.com/show.aspx?id=113180]. Regado was known as a Duke spinoff, and Robert Califf had served on its scientific advisory board since 2009, joined by DCRI colleagues Thomas Povsic and Robert Harrington.
A few months later, however, those allergic reactions were judged serious enough to shut down Regado’s Phase III study. By early 2015 the company had ceased to exist. Both Povsic and Harrington joined former Regado CEO David Mazza at Caladrius Biosciences, whose cell-based treatment for ischemic cardiac damage may well come up for approval during Califf’s tenure. Portola’s and Perosphere’s antidotes are sure to do so, and perhaps Portola’s betrixaban as well.
An obligation to report?

Names like Portola, Regado, Caladrius and Perosphere have seldom come up in discussions of Califf or DCRI. These companies don’t turn up in a search of the Sunshine Act database, which only tracks payments on behalf of drugs that are already FDA-approved. Often these days it’s established Pharma giants that will market the drugs and deal with any problems they cause, while the startups that handled the clinical trials may fade from view.
It’s unlikely the Senate would consider a seat on a pharma board to be a black mark for Califf, and certainly there is no shame in working on a drug like REG-1 that didn’t pan out. However, the results of such “failed” trials are just as important as successful ones, and sometimes more so. In recent years Califf has spoken and written extensively on the need for transparency, data sharing and the importance of trial registries like ClinicalTrials.gov. He’s also been asked to serve on both government and private task forces to fix a troubled clinical trial system.
So it’s worth asking: What about the trials for Regado’s REG-1 and REG-2? The initial good news about the drugs was discussed in several journal articles, but no results were ever posted on ClinicalTrials.gov. The results on the 3232 subjects in the terminated trial were never posted, and the registry says only that the trial is on “clinical hold.” The outcome was reported at a March 2015 cardiology conference, but not in any articles to date. A similar shortage of results seems to plague Portola, as well as DCRI studies of an Alzheimer’s drug and its work with Perosphere.
The brave new world of faster trials
The stakes could not be higher. In the coming year the US Senate will complete work on a total overhaul of the drug approval process, known as the “21st Century Cures Act.” Faster trials, lighter regulation and a bigger role for industry, all Califf watchwords, are central to the bill. The FDA has also approved two expensive new biologic drugs to manage cholesterol (PCSK-9 inhibitors), whose safety and real-world impact on health need to be closely watched. Califf served as advisor to the makers of both.
The fight for open data access, in the wake of Study 329 and countless other scandals from Vioxx to Avandia, may be even more important. Here again, the news is strange. Bristol-Myers Squibb has announced its own “transparency” initiative in which an Independent Review Committee will oversee sharing of clinical trial data with selected researchers. The “independent” committee will be set up by the DCRI, and consist entirely of Duke faculty.
It’s all part of a Strategic Partnership between BMS and Duke which was publicly celebrated at this 2014 event featuring Califf. [https://dcri.org/events/materials/bms-day/Duke_BMS_mailer_14FEB2014%20-2.pdf]. The brochure has all the feel of a wedding announcement. Should any doctor, even one as wise as Robert Califf, be asked to monitor and report on his own spouse?

Great sleuthing!
I have to comment that yesterday I was leaving a Thanksgiving visit in Raleigh NC heading home. Shortly after passing the Duke Exits on I-40, there, rising from the pines along the Interstate, was a gleaming spire – Quintiles, the biggest ever CRO.
Connections?
Oh man. Quintiles is connected to Everything.
Once again a magnificent and extremely informative piece of research.
Thank you for posting. Have been following heart meds for several years – my husband has been on many of the most lethal ones – some stopped his heart, some lowered his blood pressure so much he would collapse (regularly). His jaw is still dislocated, his teeth don’t meet, his head is off its axis. All meds were improperly prescribed. All had adverse effects which ‘needed’ other meds to control them.
Very simple afib was medicated to severe heart failure. He used to beg me not to force him to live.
I’ve been ridiculed and vilified by cardiologists for not allowing him to take the new anticoagulants. I’ve also been able to wean him off everything except a mini dose of digoxin and warfarin. The digoxin will be gone soon. In February 2015 he was told the only hope for his survival was an implantable cardiac defibrillator – otherwise he “could drop dead any second”. When I said he was feeling better, we were told “people often feel better just before they drop dead”. By July 2015 he was told his heart had improved so much he was no longer a candidate for any procedure. He’s still physically very weak. And no – he can’t get cardiac rehab because he hasn’t had a heart attack.
Hard to prove death by heart medication – just as evil and abused as psychiatric meds. A friend Carolyn Dean MD ND writes there is no heart failure – there are failed doctors.
http://cardiobrief.org/2015/11/30/new-questions-raised-about-rocket-af-pivotal-xarelto-trial-chaired-by-califf/
Thank you for the link, Maie! I had seen the new report about the malfunctioning blood-test device in the Xarelto trials — but most coverage had implied that it was just globally unreliable. Readings wandering all over the place.
However, if I read Larry Husten’s story right, the device was consistently giving LOW readings. Which means the doctors would have often responded by giving TOO MUCH warfarin.
Which would demolish one of the only “positive” findings that all the experts seemed sure of — fewer brain bleeds on Xarelto (though not fewer deaths or serious injuries). The official story is now that Xarelto causes “fewer brain bleeds but more GI bleeds.” And since we are all more afraid of bleeding in the brain than bleeding in the gut, Xarelto gets a powerful “safety” selling point.
I thought that selling point to be exaggerated or oversold — but maybe it’s truly a load of you-know-what. Wow.
And THANKS for sharing your husband’s travails! All the best to you both. It’s terribly common for people to be double- and triple-medicated for the effects of their “life-saving” heart drugs. Especially dizziness from blood-pressure drops — which in older people is easily mistaken for some sort of “general debility of aging,” with truly sad results.
The emotional reactions of a patient’s and their loved one(s) to known and suspected treatment related deaths should definitely be part of discussions about “The Ghost Of Research Future”. The fatal shooting of a prominent Boston cardiac surgeon by her son comes to mind.
https://www.bostonglobe.com/metro/2015/01/22/alleged-shooter-blamed-medication-for-mother-death-brother-says/JD5rh5tsCAua4FwFrAOFvJ/story.html
The Boston Globe reported in detail , per a witness who stated that the son/shooter confronted the doctor in an exam room, insisting he pull up info on the internet about the drug the son believed caused his mother to hemorrhage to death. According to the witness and patients in adjacent exam rooms, there was no “shouting”. The doctor and his deceased patient’s son were alone about 25minutes before shots were heard.
The shooters brother claimed that the *straw* was the autopsy report. Pulmonary bleeding was noted as cause of death, though this was not initially suspected. Post op, the surgeon ordered a “drug” that the shooter researched and determined was the cause of the fatal bleeding. Details about the specific drug were not reported, and we will never know how Dr. Davidson attempted to explain his choice of treatment to the man who fatally shot him and then himself.
Ah.. If I may jump in here…remember the 60 Minutes piect called “Deception at Duke”?
Is anyone aware of that Anil Potti cancer fraud scandal at Duke?
Here is the back story:
Califf was the top dog at the time, and a young resident researcher named Bradfor Periz alerted Duke brass (including Califf) about Potti research problems.. “the data was questionable” VIA EMAIL. But (via email) top brass wanted to hush up the young assistant.. (all captured on duke email) It remained hushed until a Texas group also found the data to be unreliable. there was big rebuttal from duke and the bogus studies continued on unsuspecting patients.. finally it all came out, the data (under Califf watch was bogus)
This all came to light early in 2015 when the medical malpractice involving that same fraudulent research, was set to be in front of a judge. (Jan 2015 if I remember right)
Just days prior to the first appearance, one of the “lawyers got the flue” and it all was postponed.. Then it was soon postponed until Fall 2015.. ? wow really?
Well (remember now, Calif was already on his way to the FDA via Oboma appointment) The Bradford Perez emails were published in “The Cancer Letter”..early 2015
After years of delay and legal maneuvering by Duke to prolong, avoid paying research victims and families.. within days of the Cancer letter making public the emails exposing Califf and other top brass involvement in the research fraud cover-up. Duke quickly and silently settled out of court,
By Summer 2015 it was all silenced, media coverage was zero.. and Califf had much to smile about (see recent photos).. Califf and Due University Hospital got off scott free for involvement in the largest research scandal 60 Minutes already reported on.
And yet no one even mentions this in the conformation hearings.. THAT is concerning!
Any typos or grammar errors, I take full credit for, but the story is rock solid facts.
Expanding Danny..
“Jan 2015 Oboma finds out Califf was directly involved in Duke University cancer research fraud “cover-up” by S. kalicky
http://shellyskalicky.blogspot.co.uk/2015/01/duke-university-hospital-cover-up.html
http://www.wral.com/news/local/story/10104999/
Wikipedia’s ‘public’ background
Heading:
This article may be unbalanced towards certain viewpoints
https://en.wikipedia.org/wiki/GlaxoSmithKline
The results of the RECORD study were readjudicated in 2013 by the Duke Clinical Research Institute, in an independent review required by the FDA. The Duke group confirmed GlaxoSmithKline’s reported conclusions that Rosiglitazone does not increase cardiovascular mortality or morbidity.[102] In June 2013 an FDA advisory committee reviewed the readjudication results, and 20 of the 26 committee members voted to remove or reduce the restrictions of the risk evaluation and mitigation strategy (REMS) that had been previously imposed by the FDA.
In November that year the FDA lifted the restrictions it had placed on the drug.[103] The boxed warning about heart attack was removed; the warning about heart failure remained in place.[98]
https://en.wikipedia.org/wiki/Robert_Califf
Relationships with the pharmaceutical industry
Califf worked very closely with pharmaceutical companies at the Duke clinical trials center “convincing them to do large, expensive, and, for Duke, profitable clinical trials.”[9] He was a paid consultant for Merck Sharp & Dohme, Johnson & Johnson, GlaxoSmithKline, AstraZeneca, and Eli Lilly per ProPublica from 2009 to 2013. The largest consulting payment was $87,500 by Johnson & Johnson in 2012, and “most of funds for travel or consulting under $5,000”, which has been called “minimal for a physician of his stature”.[10] From 2013-2014 he was paid a total of $52,796, the highest amount was $6,450 from Merck Sharp & Dohme, followed by Amgen, F. Hoffmann-La Roche AG, Janssen Pharmaceutica, Daiichi Sankyo, Sanofi-Aventis, Bristol-Myers Squibb and AstraZeneca.[11] He was the Director of Portola Pharmaceuticals, Inc. from July 2012 to January 26, 2015,[10] Advisor of Proventys, Inc., Chairman of the medical advisory board of Regado Biosciences, Inc. and has been member of the medical advisory board since June 2, 2009, and member of the clinical advisory board of Corgentech Inc.[12] Forbes wrote that his close ties to the drug industry were the reason for him not being nominated for the FDA Commissioner position in 2009.[9]
Johanna,
Just one little correction in this *heart stopping* thriller-. Monitoring patients on warfarin (coumadin) involves frequent blood tests to determine anti-coagulation effects of the drug. Usually every few weeks, once a patient is stable, the lab test of choice is an INR. There is no quantifiable *blood level* of Warfarin that indicates therapeutic effect. The important message here is that people taking warfarin or any anticoagulant are usually more at risk for excessive bleeding- if their INRs run *high* patients are encouraged to check stool samples with hemocult test to rule out GI needing, and to have routine hematocrits done to rule out anemia. Stands to reason that any clinical trials of these drugs would entail very careful monitoring of patients for complications that are potentially life threatening–
I worked in Coronary Care Units and /critical care ICUs in the 80’s in the Washington DC metro area– mostly at academic medical centers. After 5 years, negotiating the *intense* learning curve; mastering high- tech invasive monitoring and tedious titrating of very toxic drugs, I was totally burnt out by the undeniable correlation between *life saving* medical breakthroughs and inhumane treatment of critically ill patients. Just as there were nurses who became so mesmerized with numbers on monitors and forgot to even assess their patients, there were doctors who were enthralled by the lure of becoming experts at performing invasive procedures. They referred to themselves as “line jockeys”, prima donnas in critical care settings– more like “one-trick ponies” imo, they were excessively well paid. Their mantra was– all for purpose of *saving a life*– or sacrificing a life to save another life?
The culture developing in lock step with amazing medical technology violated Kant’s ethical principles, which were the foundation for ethical medical practice. The human being, the patient was no longer the “end”, to be considered first and foremost in all matters of treatment interventions, but had become a “means” to an end. The phrasing of *that end* was cloaked in altruistic ideals and garnished with a very high price tag.The marketing research went something like this, I think: How much is *your* life worth– ? Does it matter who or what was sacrificed to save *your* life? The thriving market for the latest and most astounding medical breakthroughs technology and drugs attests to the resounding answers to those two questions.
What is missing in these horrifying reports of scandalous behavior by prominent men of medicine and science, is the meaning it all has at the end of the day to our political leaders and the public/health care market. I’ve witnessed it in the stoic demeanors of former colleagues, justifying their pivotal role as life savers– all have experienced the *necessary* evils to save the next person, whom they believe may be themselves or their loved ones. No one really questions the nature of what is filed away as collateral damages.
Scandalous reports of the abject negligence shown for human safety and well being fall short of achieving public outrage for many reasons, I’m sure. But none is quite as troubling as the complacency with which the medical profession, whom most people still feel a need to trust, whose *experts* assert that their *ends* justify their *means*. And they have the means to make it appear– just so.
People who still value quality of life are less susceptible to promises of prolonging their inevitable death. People who trust their own instincts and care givers/providers who practice with compassion for the humanity of their patients are rare. Rarer still are those who have faith in their own humanity and speak out in a sincere effort to re-humanize health care. This is a re-education effort, replete with formidable obstacles — like; the fear of death.
At this time in our history, doctors still appear (to most people) to be our last hope–. They aren’t breaking ranks to steer us in another direction– who amongst us can afford to evoke their ire? I think the number is still quite — inadequate.
http://www.ctti-clinicaltrials.org/files/documents/Monitoring-Landray_IntroCTTI.pdf
http://www.telegraph.co.uk/technology/news/11892021/The-super-laboratories-bringing-the-UK-to-the-forefront-of-life-sciences.html
https://www.ctsu.ox.ac.uk/about-ctsu/documents/independent-research
http://www.nih.gov/news-events/news-releases/nih-forms-team-experts-chart-course-presidents-precision-medicine-initiative-research-network
Heck – lots of links – the product of iphone googling.
What a brilliant piece of research and trail-following. It makes riveting, if spine-chilling reading. Thanks Johanna.
Reading it, I was struck with curiosity about our home-grown, UK, all-round expert on everything biomedical – one Rory Collins. (In)famous for his tireless, altruistic work on – well, sort of everything related to the human body: brain, genomes – but most people know him for his statin research (and links to Pfizer). Too long a story to relate here – but – I googled his name plus Duke University. Lots of websites popped up – he would appear to be closely connected to Duke – and presumably all the other shysters.
So, maybe our beloved Sir Rory Collins is also the Ghost of Research Future on this side of the Pond. Certainly his Clinical Trials Centre is pretty much wholly funded by industry – although you have to dig, as usual, to discover that.
Thanks for a great piece of work
Amazing article. I researched Nuedexta to treat PBA (Pseudobulbar) because it’s one of the strangest disorder’s they’ve made up yet.The medication insert lists common adverse reactions incidence rate are >3%, and they are diarrhea, dizziness, cough, vomiting, ect, ect, ect. But nowhere does it list ‘Falls.’ The clinical trials not only shows common adverse rated > 5 % but ‘Falls’ was the # 1 adverse reaction. And it’s being marketed for the elderly with Dementia. Aren’t they at the greatest risk for Falls? After looking over the clinical study trials they also state “the agent was safe and relatively well tolerated when the Serious AE’s (SAEs) were reported in 126 patients, including 47 deaths” out of only 553 PBA patients, 296 completed. It’s metabolized by the P450 2D6 enzyme, but what if you lack that enzyme?
The adverse events reported under Serious Adverse Reactions and Other Adverse Reactions is 54, 15%. Isn’t this number high given the low number of trial participants in this study?
Morning, Jo…more blots on the inkpad..
Time and Recused
http://time.com/4122405/fda-robert-califf-recusals/
Faculty Connection
http://www.faculty-connection.com/about-us/
United States Office of Government Ethics
OGE Form 201
http://www.oge.gov/DisplayTemplates/201_Form/AutomatedLanding.aspx?id=2147484576&query=Robert%20Califf
“The difference between capture and collaboration towards improving human health is a pretty big difference”
http://time.com/3714242/candidate-to-lead-fda-has-close-ties-to-big-pharma/
•
Mickey
December 2, 2015 | 8:41 AM
Johanna,
The biggest Amen of all,
And by the way, that RxISK blog about FDA nominee Dr. Califf was a twelve on a scale of one to ten. Super sleuthing extraordinaire!…
The Super Sleuths!…
The original piece by Dr. Healy, as well as the learned comments above, all need to find their way to Senator Elizabeth Warren. If you are not a constituent of Warren, it is hard to get an email sent to her on her website. However, there may be some other way – even snail mail to her Washington or Boston office.
She is a real facts person, and, with Bernie Sanders, needs all the help she/they can get to stop the unfortunate nomination of Califf.
Well qualified response from Johanna on MIA
Johanna Ryan (MIA Author) on December 5, 2015 at 11:52 am said:
It’s similar to our battle, and plenty of us will end up fighting both.
Ghost of Research Future close to home
Fool.co.uk is a Financial Analyst
Fool.co.uk talks psychological..about Sir Andrew Witty and the future of GlaxoSmithkline
https://uk.finance.yahoo.com/news/neil-woodford-glaxosmithkline-plc-hsbc-085030795.html
Street Cred
CNBC Sir Andrew Witty/Interview
http://video.cnbc.com/gallery/?video=3000462665&play=1
And me? Well, I’m off to find me a lawyer, need more than luck to find one here in “oh-nothing-bad-happens-off-Seroxat-Sweden”!
He will probably repeat what my psychiatrist says: ssri’s may or may not have side-effects, live with it.
Wish ‘Karen Barth Menkes’ was fed up with litigations in the U.S. and decided to move here to shape us up.
Or maybe Shelley Jofree needs a new career? I bet she would be like a wirlwind in Court, rounding up GSK Corporate lawyers like frightened sheep.
Dreams aside, I Think I can give them a good fight anyhow, atleast more of a fight than doing nothing.
So when I get hung out to dry, plz send some cheer-me-ups from all of you who know the flipside of Seroxat/Paxil.
Ove. Sweden. 2015.
Update – http://cardiobrief.org/2015/12/13/rocket-af-investigators-seek-to-calm-concerns-about-trial-reliability/
From my post today on Age of Autism:
GSK Document Appears to Show Vaccine Committee Chair Used Position to Favour Own Product
http://www.ageofautism.com/2015/12/gsk-document-appears-to-show-vaccine-committee-chair-used-position-to-favour-own-product.html
The chairman of the Joint Committee on Vaccination and Immunisation (JCVI), the British equivalent of the ACIP (Advisory Committee on Immunization Practices), Prof Andrew J Pollard spoke this September at an event sponsored by vaccine manufacturer GlaxoSmithKline “Evening of Evidence/Vaccination Science to Policy: Introduction of new vaccines to the UK vaccine schedule with limited evidence of efficacy (sic): Meningococcal Group B and maternal pertussis vaccination”. Prof Pollard spoke on the subject of Meningitis B vaccine which he helped to develop and latterly seems to have superintended the process of having it added to the United Kingdom vaccine schedule as chairman of the JCVI – his talk was entitled: “JCVI decision-making process informing the recommendation for the introduction of Bexsero to the UK vaccination schedule”. Bexsero vaccine was developed by Novartis but their vaccine division was acquired by GSK earlier this year, following the approval of Bexsero vaccine by the JCVI (negotiations began within days of the JCVI approval). A deputy chair of the JCVI, Dr Andrew Riordan, spoke at the same meeting on the subject: “Evidence considered by the JCVI to recommend antenatal pertussis vaccination in the UK”. GSK also manufacture Boostrix – in fact a pertussis, tetanus and diphtheria vaccine – which is the product currently given to pregnant women in the UK, which also has an aluminium adjuvant.
Last week members of the Scottish Parliament heard how Prof Pollard apparently chaired a session of the JCVI in March 2014 which recommended Bexsero for British infants, though the committee under a different chairmanship had rejected it months earlier. It was not disclosed on the records of the committee that Prof Pollard was “named in patents in the field of meningococcal vaccines” and was contracted in continuing research relating to Bexsero….
http://www.ageofautism.com/2015/12/gsk-document-appears-to-show-vaccine-committee-chair-used-position-to-favour-own-product.html
From Elizabeth Hart
John, as you note in your post above, the Australian Pharmaceutical Benefits Advisory Committee has rejected the Bexsero meningococcal B vaccine (three times).
Despite this rejection, ‘experts’ in Australia continue to agitate for this GSK vaccine to be added to the national vaccination schedule.
An article titled “Meningococcal B vax objection a bad move: expert”, published on the Medical Observer website on 24 August 2015, reports:
QUOTE:
“A leading immunisation researcher says rejection of funding for the meningococcal B vaccine suggests the government’s advisory committee isn’t accurately estimating the cost of the disease to the community.
Pediatric infectious diseases specialist Professor Robert Booy has criticised the Pharmaceutical Benefits Advisory Committee (PBAC) for rejecting listing of MenBV, also known as 4cMenB (Bexsero, GSK), pointing out that the UK is adding the vaccine to its childhood schedule.
“Whatever reservations the PBAC has, they are not shared globally,” he says.
The third rejection of meningococcal B vaccine, which costs $140 per dose privately, was among a swag of July decisions on PBS submissions released last Friday.
The PBAC says the bid for including Bexsero on the National Immunisation Program was rejected because of the sponsor’s “optimistic assumptions” on the extent and duration of the vaccine’s effect and herd immunity, as well as lack of cost effectiveness.
But Professor Booy, from the University of Sydney and the Children’s Hospital at Westmead, says the PBAC is underestimating the impact of the disease on disability.
“I am treating patients who are still experiencing health impacts from meningococcal B more than 10 years down the line,” he says.
END OF QUOTE
Note that Booy says “Whatever reservations the PBAC has, they are not shared globally”, a reference to the UK adding Bexsero meningococcal B vaccine to the schedule.
This is what I call the ‘domino effect’, i.e. where one country adds a new vaccine product to the schedule, and then this is used to motivate others to follow…like sheep…a very useful process for the vaccine manufacturers developing massive global markets for their vaccine products, assisted I presume by the influential network of members of vaccination committees across these countries.
There are similarities here with the fast-tracked adoption of HPV vaccination around the world. The Gardasil HPV vaccine product was originally rejected by the PBAC in Australia, but was subsequently added to the Australian vaccination schedule after interference by senators and other politicians, and vested interests, with then Prime Minister John Howard insisting the Gardasil HPV vaccine product be added to the schedule in the run-up to the 2007 election.
HPV vaccination (Merck’s Gardasil and GSK’s Cervarix) was subsequently fast-tracked for children all around the world, despite the fact we have no idea of the long-term consequences of this vaccine product.
Many children are receiving up to three doses of this aluminium-adjuvanted vaccine product, and do not know they are part of a massive experiment. Any notion of ‘informed consent’ before this vaccination is a joke, particularly in Australia where vaccination is being made compulsory for children of all ages to access financial benefits from January 2016.
(For more background on the questionable international fast-tracking of HPV vaccination, see my letter to Irish senator Paschal Mooney, who has spoken out on behalf of young girls and women in Ireland suffering adverse events after Gardasil HPV vaccination. My letter to Senator Mooney, dated 4 November 2015, is accessible via this link: http://bit.ly/1PlXG08 )
Back to the Bexsero meningococcal B vaccine product, Booy says: “I am treating patients who are still experiencing health impacts from meningococcal B more than 10 years down the line…”
Apparently the PBAC rejected the Bexsero meningococcal B vaccine “because of the sponsor’s “optimistic assumptions” on the extent and duration of the vaccine’s effect and herd immunity”, so there’s no guarantee this vaccine product will prevent all further cases of this rare disease.
It seems to me this rare disease does not warrant mass vaccination, particularly as there is the potential for ‘unintended consequences’ if there is artificial interference in the natural progression of this disease, which currently poses little risk to the population.
In this regard we should keep in mind what is happening with pertussis, i.e. pertussis vaccination being implicated in the spread of the disease via vaccinated individuals.(1)
I suggest there is much that is unknown about vaccination and immunisation, and it would be useful if so-called ‘experts’ exhibited a little more humility and caution in this area.
We should also keep an eye on the PBAC in Australia, and see if there are further moves to over-turn the rejection of GSK’s Bexsero meningococcal B vaccine product…
Reference:
1. See for example FDA study helps provide an understanding of rising rates of whooping cough and response to vaccination. FDA News Release, 27 November 2013: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm376937.htm and Jason M Warfel et al. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. PNAS, 22 October 2013: http://www.pnas.org/content/111/2/787.full.pdf
Thanks David
Regarding Booy a recent disclosure stated:
“R. Booy has received funding from bioCSL, Roche, Sanofi, GlaxoSmithKline (GSK), Novartis, and Pfizer to conduct sponsored research or attend and present at scientific meetings; any funding received is directed to a research account at the Children’s Hospital at Westmead.”
http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21159
And he was cited in a 2013 GSK Press Release:
“Professor Robert Booy, who is Head of the Clinical Research team at the National Centre for Immunisation Research and Surveillance (NCIRS) welcomed the development saying that reducing “needle burden” was important in overcoming barriers to better immunisation rates..etc”
http://au.gsk.com/en-au/media/press-releases/2013/new-gsk-additions-to-the-national-immunisation-program-nip/
I just read all 231 pages of this: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/pediatricadvisorycommittee/ucm261385.pdf 2011
Two of the drugs heavily discussed were Intuniv and Lexipro – both very dangerous psychiatric drugs.
And I am so appalled at the process our FDA uses when addressing Adverse Events reported. Especially the excuses pharmaceutical spokes persons used when tearing apart all the adverse data that they make public: blaming the patient, blaming this, blaming that, but NEVER blaming the drug they are selling and making millions and billions on. I can only imagine how horrific the true number of adverse results are that are hidden, buried, and gone – that we’ll never see.
This is so outrages it defies logic when it comes to drugging our children in America. WOW! This entire FDA conference left me absolutely outraged, and speechless.
One issue brought up: When the drugs are prescribed ‘off label’ as 90% of them are how does the FDA track those Adverse Events? I didn’t understand the mumble jumble answer that was given because the entire 231 pages of information and answers provided were so well pre-planned and skewed by unreliable clinical data that I didn’t believe any of it yet these are the people (our FDA) pushing psychiatric drugs through to our children. Sometimes, fast tracking them through. This entire process is Criminal.