An instalment in the Politics of Care
In the Beginning
For nearly 200 years, concerns about individual and public Health have been a badge for progressive politics. The revolutionaries in Paris in 1790 saw healthcare as close to the most important element of the ancien regime that needed dismantling and replacing with something that would work for the people.
Fifty years later, in the 1840s, doctors played a significant role in creating socialism and communism. The health problems of workers and the clearly unsanitary state of new urban environments were put in the balance with private industries, which driven by capitalism, showed little concern for workers’ health or the environment. Industry was found wanting. A focus on individual and public health offered a way to get industry to improve working conditions, including pay, and to get governments to factor the interests of citizens into policies.
An interest in the latest techniques and the contribution these could make to efficiencies was also a badge for progressive politics. Liberal parties in the nineteenth century favored a turn to technology which led to new industries and medical developments. Liberals also favored an expansion of education.
Green New Deal or De-Growth?
Today, the changing global climate has brought Green politics into the frame for all of us. Most Greens view themselves as progressives.
The language of the arguments between those interested in, or hostile to politics that are Green or Sustainable, and between those worried about or dismissive of climate change and pollution, steers us to entrenched positions on economic battlefields. Some figure we have no option but to embrace radical change. For others, the changes sometimes proposed spell an end to life as we know it, a return to a more primitive level of society and a loss of belief in the capacities of human ingenuity, driven by market forces, to solve problems.
On the more socialist side of Green politics, there is divide between Green New-Dealers and De-Growthers. Green New-Dealers are close to classic socialists. They figure they need a seat at the table where productive techniques get brought into play – able to argue for a more Green alternative like electric cars as opposed to internal combustion vehicles. On this line of thinking, its not about shrinking the economy, it might even be about growing it.
The De-Growthers are more Luddite than communist in their belief that we need to step back from growing the economy – we need fewer cars and travel rather than just cleaner cars. We may need to let the economy shrink even if in the short term this could have dramatic impacts on all of us. The Covid pandemic has made more of us aware of the implications of growth and non-growth in a way that few could have predicted before it struck.
Controlled Green Healthcare
The Politics of Care relocates questions about sustainability into a healthcare domain in part to see whether doing this brings common ground into view.
Neither Green New Dealers nor De-Growthers mention much about healthcare other than as a consequence of a climate change that will lead to food shortages or lead to flood damage and wildfires that will lead to loss of life or lead to an air pollution that will cause respiratory diseases. These are real issues – a change in diet, pollution and physical activity could make a substantial contribution to health and minimize the number of premature deaths.
There is also a recognition of some not specifically Green issues such as containing the food industry marketing of suboptimal diets that lead to obesity with knock-on health consequences like diabetes, and the marketing by related industries of alcohol, tobacco and other hazardous products.
There is also a distinctively Green, but less often mentioned contribution to health, thatcame from epidemiological and occupational medicine studies in the 1950s of the hazards environmental toxicities pose to our health – 80% or more of cancers are likely to have an environmental origin. The warming of our climate is now so dominant an issue that it is often forgotten that these studies were in at the birth to the environmental movement.
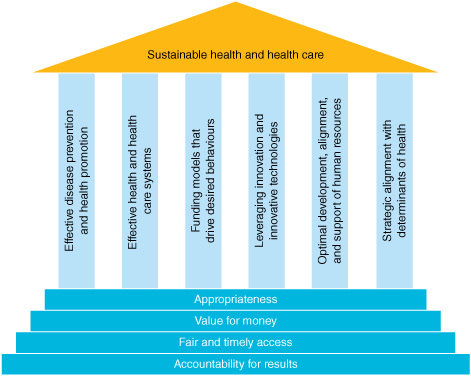
In healthcare now, those laying out Green or sustainable principles (See Lancet references below of the Canadian Alliance for Sustainable Health Care, who produced the image above) call for:
- Reduced health consumption by promoting preventive services.
- Increase in public healthcare which will better steward resources and match supply and demand.
- Reduced emissions from healthcare staff, patients, and facilities – using perhaps the rise of virtual consults as a result of Covid.
- Increase in evidence based best practice.
Sustainable healthcare needs more than this. As things stand, principles like these will do more harm than good.
The reason we end up with principles like these is that the focus to date has been on classic productive industries, the chemical, petrochemical, armament, and other enterprises whose products lead to climate change and environmental degradation. For decades, progressive politicians have sought a seat at the table where decisions about production are taken confident the public will vote for them because many of us view our current problems as stemming from an industrial and polluting revolution – with some of the rest of us figuring well maybe but it also produced wealth and gave us cars.
The Technical Revolution
From a 2021 vantage point, the industrial revolution looks like one element of a more encompassing technical revolution that began just before and triggered the French revolution whose greatest symbol was the use of a new medical technique – the Guillotine – see Shipwreck in Maastricht.
From 1950 onwards, the manufacturing industry born around that time shed jobs, just as agriculture sector that it had replaced had done around 1840. The proletariat have vanished just as the peasants did – they are no longer there to be a revolutionary class.
Close to 80% of us are now employed in service sectors, of which healthcare is the biggest component. Our politics do not reflect this.
The service sector uses rather than produces chemicals and plastics. It leaves emissions in its wake. Making healthcare sustainable, however, is about more than reducing the use of these materials and the level of emissions from healthcare staff or patients travelling in cars to hospitals.
Service sector management practices, which themselves are part of a bigger technical revolution that took place 200 years ago, toxify our environment.
One of the greatest ironies is that these management practices have been billed as bringing an entrepreneurial spirit and private sector efficiency to health services and to education.
These practices in fact are algorithmic in nature and oriented toward finding operational or technical solutions to our problems – oriented to solutions that have produced the problems we now have in both the productive and service sectors. The latest algorithms foster demand rather than contain it.
Demand can only be contained through an exercise of discretion, but we have to work out a way to value discretion. Discretion cannot be operationalized in a manner that makes is ‘scalable up’. It is local rather than global. And this localisation starts with the fact that we have a body, even as the latest technique lets me communicate with people on the far side of the world. See Annemarie Mol’s Eating in Theory.
The service sector will not produce techniques like the plastic eating bacteria that might help with pollution, but it is key to producing a recognition of the need for judgement and a respect for the complex nature of technique. This is becomes most clear when we are faced with the adverse effects of a drug.
Sacraments or Poisons
Until recently, medicines were widely viewed as poisons from which good can be brought if used judiciously. All techniques share this character – they inevitably cause harm but good can be brought from their use if that use is judicious.
Instead, we now view medicines and techniques in general as magical. Within health algorithms, predicated on the idea that the magic lies in the latest technique, have turned healthcare into what are better called health services. When Joe Biden or any politician says we will Follow the Science, the will invariably Follow the Algorithm.
Science and politics don’t mis easily. Science generates questions rather than closes them down. Politicians want policies which people will follow without question. Invoking Science is saying there are experts and you and even I, JB, need to shut up when they speak.
In healthcare we have reached just the opposite point – a lot of the experts have lost a lot of their credibility and listening to an increasing number of them is dangerous for our health.
Healthcare needs a recognition that the magic lies in us. Locating the magic in us rather than in techniques is not a matter of inhibiting production or services, nor a matter of locating our problems in some original corporate sin but of harnessing production and services so that they serve us rather than having us serve them.
It’s a matter of producing health rather than consuming health service products.
We need an appreciation of the role our judgements make in shaping whether techniques work for us, not just in the short term, but overall. Our naïve embrace of technique undermines the current principles offered by Green advocates:
- Preventive medicine has been commandeered by pharmaceutical companies and it is now a major driver of health service use.
- Public Services now also drive the current growth in health services – reducing medication burdens has become a privilege of wealth. Waving a public health service wand over our difficulties will no longer work the way some hope.
- The virtual consults that stem from the Covid pandemic may reduce emissions but at a cost of increasing the technical character of health services, increasing services overall and reducing the close personal contact without which there can be no Care.
- Evidence based medicine is almost totally controlled by industry – see related posts.
The Politics of Care
The politics outlined on this site invites everyone concerned with sustainability whether from Green or traditional political parties, or none, to engage with changes in the climate of healthcare and ask – what would truly sustainable healthcare look like? A follow up question is does sustainable healthcare shed a light on global climate change?
There is an urgent need to grapple with the following facts:
- Life Expectancy has been falling for 5 years before Covid.
- Our falling life expectancies can be tied to the number of drugs we now take. To get what we want from treatment we should ideally be on no more than 3 medicines. Our values, not an algorithm, must determine what these 3 are.
- Our participation in genuine conversations about our health has been compromised as the literature on our drugs is entirely ghost-written. The greatest concentration of Fake literature on Earth has, for 30 years, centred on the drugs our doctors give us.
- Our conversations are further compromised by a lack of access to the data from the clinical trials on these drugs. This enables companies to spin their drugs as safe and effective when the raw data (if accessed) shows them to be ineffective and unsafe.
- For similar reasons, healthcare professionals cannot have genuine conversations with regulators or the managers of health services.
- This allows corporations to bring us health services that deliver us problems we never knew we had for which their drugs are the answer. Nowhere is this truer than in preventive medicine.
- This corporate approach is making the Healthcare climate toxic and lowering life expectancies.
- Just as the effects of accidents like Exxon Valdez increase GDP, so drug induced disabilities now sustain services rather than us.
- In addition to a corruption of preventive medicine, there is a more recent growth in wellbeing markets. This adds to a consumption of health products and to our medication burdens. It also imports identity politics into the health domain, further fracturing our abilities to find common ground.
- Healthcare cannot be an exercise in consumerism. Poisons and techniques that can backfire are not consumer items. Care must be relationship based. It hinges on wisdom and humanity rather than marketing, and algorithms.
- Green principles see the hazards of alcohol and smoking but fail to see a link to drugs that are prescription only because they are more hazardous than alcohol or nicotine.
- Medical drugs have been transformed from wine into sacraments – substances that can only do good. They are the exemplar of all techniques in that the magic needed to use them to good effect lies in us, but we are tempted to view the magic as lying in the technique.
- Salvation lies in participation (production) rather than consumption.
Boundaries
At the moment, the powers that be are very comfortable with the development of Green politics. Any proposal from an opposition that invokes a need for innovation (as in CASHC above) or adhering to the best possible evidence is not a problem. There is no pressing need to do anything to manage the proposers no matter how revolutionary, or anti-establishment they may seem, or how (to use the current status quo jargon) disruptive they may seem.
While they invoke innovation and new techniques, there is a good change they will control themselves. Token expressions of alarm from time to time may be useful to keep them on the right track – the same track.
For the rest of us there is a need to find the boundary between being part of a controlled opposition and a force for real change. This was brought home to me yet again lately when I was described as part of the controlled opposition.
References for Politics of Care:
Shipwreck of the Singular: Healthcare’s Castaways. Samizdat Writers Cooperative Toronto.
References for standard Green healthcare positions:
See CASHC above and the following from the Lancet Planetary Health available for download.
Barlow P, van Schalkwyk M C, McKee M, et al. COVID-19 and the collapse of global trade: building an effective public health response. Lancet Planet Health 2021; 5: e102–07
Dasandi N, Graham H, Lampard P, Mikhaylov SJ. Engagement with health in national climate change commitments under the Paris Agreement: a global mixed-methods analysis of the nationally determined contributions. Lancet Planet Health 2021; 5: e93–101
Fu-Chun MC. Accelerating towards net zero emissions: the most important global health intervention. www.thelancet.com/planetary-health Vol 5 February 2021 e64
Hamilton I, Kennard H, McGushin A, et al. The public health implications of the Paris Agreement: a modelling study. Lancet Planet Health 2021; 5: e74–83
MacNeill A, McGain F, Sherman J Planetary health care: a framework for sustainable health systems. Lancet Planet Health 2021, 5 e66-69.
Half-zipped at the fire…
The Healthcare World According To Andrew Witty
https://pharmaintelligence.informa.com/resources/product-content/the-healthcare-world-according-to-andrew-witty
Two and a half years after stepping down as CEO of GlaxoSmithKline PLC, Sir Andrew Witty returned to London to talk about life after big pharma and his views on the US healthcare system where he has become a leading figure as head of UnitedHealth Group Co.’s pharmacy benefit management and health services arm Optum.
Eyebrows were raised when Witty, an advocate of the need to change pricing models when at the helm at GSK, switched sides in July last year to run Optum. In addition, last week, he was named as the new president of UnitedHealth, the largest private payer in the US which reported whopping revenues of $60.4bn (+7%) in the third quarter.
Speaking at the Pharma Integrates conference in London on 18 November, Witty told attendees that the US was spending almost 20% of its gross domestic product on healthcare and patients are becoming more exposed to higher premiums and out-of-pocket expenses. “This has driven anxiety about affordability,” he said, claiming that Optum is leading the charge in pushing for a simpler and more cost effective health system.
This involves aligning pharmacy, medical and behavioral health needs, using data to enable end-to-end management of care, according to Witty. The key element of this involves moving much care out of hugely-expensive hospitals and he spoke about the convenience of patients getting treatment at retail stores in shopping malls rather than travelling to out-of-town centers.
“I got my Shingrix in Target,” Witty revealed, referring to GSK’s shingles vaccine and the US retail major. Shifting the site of care cannot just lower costs but can also improve clinical outcomes, he noted, with the likes of home infusion services.
Witty said that the digital space in healthcare was clearly of interest: “The key is not to get lost in the noise [and] we keep declaring victory extremely prematurely.” He stated that he was “very sceptical” when he hears “digital solves” but is “a big fan” of a digital element adding value.
Witty believes there is a place for behavioral health apps, including those based on ‘nudge’ theory, that propose subtle changes to the patient’s environment that can boost compliance. He noted that Optum has spent over $1bn on incentives, “so if you get a flu shot or you lose weight, you have a reward.”
The digital revolution, be it through wearables or insideables, needs to be led by clinical informers, Witty stressed, adding that the personal touch is still vital. He said that targeted human intelligence complemented with artificial intelligence is both a challenge and an opportunity.
By using data personalized to a patient, analytics will help determine not only a “best action” but also it needs to predict what the individual’s next best action will be, Witty said. Using these analytical tools works better when the patient is inspired to invest in their own wellness, he added, noting that behavioral health programs run by Optum with some of the poorest communities in the US with mental health problems who had considerable compliance issues have seen a marked in adherence to medicines.
When it comes to the role of digital technology in healthcare, Witty said that “the UK is a phenomenal position,” given its single-payor status where all citizens are provided with access to treatment. Having all the data in a common source makes sustainable and systemic chance more likely to succeed in the UK than most countries, he pointed out.
Thank you! As always brilliant analysis.
I have to confess that I’m worried for things are escalating quickly.
The colour of money is definitely not green. Here;s A snippett from a stomache churning document well worth a read but which ‘health service users’ know little about. Is the NHS fatally wounded ?
https://www.nhsforsale.info/private-providers/the-practice-group-ltd-new/
Operose Health was formed in January 2020, when Centene Corporation, a large US health insurer, brought together its subsidiaries in the UK – The Practice Group (TPG) and Simplify Health. The Practice Group was acquired by Centene in 2017.
In February 2021, Operose Health took over the large GP surgery company AT Medics.
The takeover of AT Medics was finalised 10 February 2021, when the directors of AT Medics Limited resigned and were replaced by Samantha Jones (CEO of Operose and ex-head of NHS England’s new care models programme), Nick Harding (Director of Operose and formerly Senior Medical Advisor to NHS England for Integrated Care Systems and Right Care) and Edward McKensie-Boyle, Chief Financial Officer of Operose.
Operose states in the 2018 annual accounts that there has been a change of approach to its market strategy and as a result a number of decisions were made “to exit contracts that have not historically fulfilled profitability targets, or do not fit with growth strategy.” At the end of March 2019, the company exited the Surrey Borders Partnership Trust CAMHS contract, at the end of June 2019, it exited the Surrey Borders Partnership Trust CFHS contract and in July 2019 it divested its complex care division.
In December 2018, Centene UK Ltd appointed Samantha Jones as its CEO; Jones was head of NHS England’s director of new care models from 2015 to 2017, and previously chief executive of Epsom and St Helier University Hospitals and West Hertfordshire Hospitals trusts.
By mid-2010 The Practice had 16 contracts with GP surgeries. The company then underwent a phase of rapid expansion through the acquisition of other companies.
In November 2010, it acquired the surgery business of Chilvers McCrea (over 30 surgeries), in April 2011 the surgery business of United Health UK (six surgeries). More recently in May 2016 The Practice acquired Phoenix Primary Care Limited (12 GP surgeries.). At the time of the acquisition, Phoenix had 58,000 registered patients.
In April 2017 The Practice launched a new complex care division to provide home-based complex healthcare services to people with significant health conditions, long-term illnesses or disabilities. The division was known as TPG Complex Care. In May 2017 it launched its opening at headquarters in Telford.
Financials
As of December 2018, MH Services International (UK) Ltd (Company Number: 10926787) became the parent company to Operose Health UK Ltd and Operose Health Group Ltd (formerly Centene UK Ltd).
MH Services International (UK) Ltd is a wholly owned subsidiary of MH Services International Holdings (UK) Ltd (Company Number: 10926063.)
According to Companies House, MH Services International Holdings (UK) Ltd has filed accounts to year end December 2019. For year end December 2019, revenue was £23.8 million (2018: £34 million) and it reported a loss of £15.3 million (2018: loss of £23.7 million).
According to Companies House, the last accounts filed for MH Services International (UK) Ltd ran to year end December 2018 recorded revenue of almost £34 million, however it recorded a loss of £23.7 million, compared to a loss of £10.5 million the previous year.
The accounts show that the group derives all its revenue from NHS contracts, including GP surgeries, community healthcare services and consultancy services.
Investors
Operose Health is 100% owned by the US company Centene Corporation. Centene Corporation is a Fortune 500 company listed on the New York Stock Exchange.
Centene is a publicly-traded company that acts as a major intermediary for both US government-sponsored and privately-insured health care programs. Centene works with Medicare, Medicaid, and The Health Insurance Marketplace System, as well as traditional commercial insurance. The company has over 30,000 employees across the USA and operates health plans that serve 2.9 million members in 24 US states.
In January 2020 Centene Corporation loaned MH Services International Holdings (UK) ltd the funds for an investment in Circle Health Holdings. The investment means that MH Services International (UK) Ltd has a total voting interest of 40% in Circle Health Holdings and by extension in its recent acquisition of BMI Healthcare. Centene’s investment in Circle via MH Services International Holdings (UK) Ltd is now valued at £67.3 million. The accounts of MH Services International Holdings (UK) Ltd note that the investment gives the Group significant influence, but not control over Circle Health.
The Practice Group Ltd struggled financially since its beginnings and, according to accounts filed with Companies House, never made a profit. The company has been shored up with money from venture funds and investors, and was finally acquired by Centene Corporation in 2016.
The company’s first major investor was the venture capital fund MMC Ventures Ltd. The initial investment of £865,000 was in 2006. This was followed by a further £1.4 million in October 2007 and £1.1 million in July 2007.
Contracts
Operose Health, as The Practice Ltd, has contracts to run GP surgeries, with 20 listed on its website, plus the Birmingham walk-in clinic. The company runs ophthalmology services at 10 sites and one dermatology service.
The AT Medics acquisition is a significant addition to its portfolio as it has 49 GP surgeries across London. Most recently, in February 2020, the company was the most successful bidder on the contract “PRJ736 — London APMS GP Contracts”, winning six of the 15 lots on offer, with contracts running for 15 years and worth a total of just over £121 million.
In November 2020, ContractFinder, the government tendering database, reported that Operose was one of 67 suppliers awarded a place on the NHS framework contract NHS Increasing Capacity worth in total £10 billion. The framework runs until November 2024.
As Centene, the company came to the attention of the media in the UK in 2017 when it was given a sub-contract by Capita for part of a contract for the development of an accountable care system (ACS) in Nottinghamshire.
The original one year £2.7 million contract was awarded to Capita by Nottingham and Nottinghamshire STP.
In September 2018, Centene was listed on 9 of the 10 lots on the Government’s £300 million framework contract for digital technology in the NHS. The ten lots cover helping health regions to integrate and improve services using technology and external consultants. NHS England has estimated that £300m of business will be done through the framework over four years. The framework is known as Health System Support.
In June 2012 TPG relinquished a contract to run the Brandon Street surgery (also known as Belgrave Health Centre) in Leicester. TPG had failed to recruit permanent GPs for the surgery and a succession of locums was used. Patient complaints noted difficulties in getting through on the phone and making appointments, rude staff, and the use of locums which meant medical history had to be gone over again and again in the limited appointment time allowed. TPG took over the running of this surgery and three others in Leicester in 2010 for five years under a £5 million contract.
In April 2012 TPG closed its Camden Road surgery in London, which it had bought from United Health, when its lease ran out. There was an outcry from patients who had been given very little notice of the decision. Both United Health and TPG were accused of running down the surgery and there was considerable use of locums.
In 2011 The Practice was given notice to terminate its contract for the St James medical practice in Handsworth, Birmingham, in December 2011. The Practice Plc noted that “The type of contract we have is a ten-year fixed term and the PCT have the option to terminate at the mid-point which they have chosen to do. This coincides with the unexpected termination of our lease by Vitality which means we will have no premises to practice from after December 31.” The Vitality Partnership is a GP partnership across five practices in Birmingham.
There was further controversy in January 2016 when TPG announced its intention to terminate its contract for five surgeries in Brighton and Hove following a funding dispute. This left the future care of 11,400 people in doubt. Patients have since been dispersed to other practices and only one practice, the one serving the homeless community, has been recommissioned.
Accountable Care strategy
A major concern in the UK is based on Centene’s record in the area of accountable care systems, in particular its involvement in the accountable care company Ribera Salud in Spain.
Centene originally owned 50% of Ribera Salud, a Spanish Healthcare company. As of July 2019, Centene now owns 90% of Ribera Salud, after purchasing a stake from Banco Sabadell. Ribera Salud is known for pioneering the development of the public/private partnership model of healthcare in Spain. Centene notes on its website that it will save the NHS money and references its “experience with Ribera Salud in Spain” which it says “is recognised by governments across the world as an example of an effective model of care……[that] produces better results for the patient at less cost to the government.”
In an article in HSJ in June 2017 Ribera Salud’s model of integrated care is also lauded and its capacity for reducing costs praised. Ribera Salud developed an integrated model of healthcare at the Alzira hospital in Valencia, which has become known as the Alzira model.
Under this model, Ribera Salud received a capitated budget from the regional government over a 15-year contract. Ribera Salud must then provide free healthcare to a defined geographical population. In return, it retain profits of up to 7.5% of turnover, but anything above this is returned to the government. The model encompasses hospital services and primary care. According to the HSJ article, the Alzira model spends 25% less than government run hospitals in the area and has been hailed as a major success for integrated care. The Alzira model has been replicated in other areas within Valencia and also in Madrid.
What neither Centene nor the HSJ article mention, is that Ribera Salud is currently under police investigation and there is a process underway in Valencia to take the hospitals back into public control. The Spanish newspaper El Pais reported in November 2016 that Ribera Salud is under police investigation for fraud, including overcharging, and issues with sub-contracting. In March 2017, El Pais reported that the regional Valencia government is to do a “reversión de la sanidad privatizada” literally a reversion of privatised health, under which as the contracts or “concessions” come to an end, the hospitals will be transferred back into public management.
One of the major election campaign promises for the Green/Socialist/Podemos coalition government in Valencia, which won the regional election in 2015, was stopping and reversing health service privatisation. There have been significant problems with a lack of oversight of the “concessions” given to Ribera Salud, with no effective control, nor checks on the quality of its service, nor in any financial matters, according to Ximo Puig, President of the regional government in Valencia.
We are researchers and journalists investigating and campaigning in support of our NHS.
Trust us we are men we know more about your female bodies than you silly women. And if you challenge us we will pass you around like a parcel until you begin to think youre mad – and prove it by getting you to see our pal the psychiatrist who is the expert on women’s mental illnesses – trust him you need antidepressants – amongst other horrendous accounts.
https://www.bbc.co.uk/sounds/play/live:bbc_radio_4 Today 10am -11am
One of the of the best episodes of Woman’s Hour presented by Emma Barnett with evidence of how woman are still being mistreated and harmed by the misogynistic attitudes of male medics (There are fewer women who have swallowed their masters’ attitudes)
Call for women to submit their experiences to a consultation (we know what the limits of those are but it seems worth doing)
https://twitter.com/BBCWomansHour/status/1402552486523703296?ref_src=twsrc%5Egoogle%7Ctwcamp%5Eserp%7Ctwgr%5Etweet
GOV.UKShow or hide search
Open consultation
Women’s Health Strategy: Call for Evidence
Updated 1 May 2021
Executive summary
We are seeking your views to help inform the development of the government’s Women’s Health Strategy.
This call for evidence is seeking to collect views on women’s health. It will run for a period of 14 weeks and is open to everyone aged 16 and over.
The easiest way to participate in the call for evidence as an individual is by completing the public survey.
We also welcome written submissions from individuals or organisations who have expertise in women’s health, such as researchers and third-sector organisations.
This consultation closes at 11:45pm on 13 June 2021.
Ministerial foreword – Nadine Dorries
As Minister of State in the Department of Health and Social Care, one of my key priorities is women’s health. Throughout my time as Minister, it has become clear that there are some key themes which cut across different areas of women’s health, and on which we must take action.
We know that damaging taboos and stigmas remain around many areas of women’s health, which can prevent women from starting conversations about their health or seeking support for a health issue. When women do speak about their health, all too often, they are not listened to. Independent reports and inquiries – not least the First do no harm report and the Paterson Inquiry report – have found that it is often women who the healthcare system fails to keep safe and fails to listen to. We absolutely must change this.
See new Tweets
Tweet
BBC Woman’s Hour
@BBCWomansHour
·
1h
Have you ever been disbelieved by your doctor?
@ElinorCleghorn
talks about the long and shocking history of women being cast as unreliable narrators of their own health https://bbc.in/3w9FvEY
Sarah Tonin – their little joke…don’t want to be a party-pooper but why Send-Up Serotonin?
its not often that the mechanisms of SSRIs are discussed so we have a light-hearted laughy-jokey type programme like this when for some the SSRIs have proved fatal –
Twin brother Chris van Tulleken supports Antidepressant Risks
https://www.madinamerica.com/2021/05/antidepressant-risks-new-website-informs-shares-personal-stories/
Xand goes for a different approach.
Serotonin
https://www.bbc.co.uk/programmes/p09cd8b9
Chris and Xand are employed as TV doctors, as educationalists, as the voices with suggestive overtones, as a composite version of truth so is Xand doing us a favour or making too little too light…
The BMJ
Access thebmj.com – The BMJ logo
Why we petitioned the FDA to refrain from fully approving any covid-19 vaccine this year
June 8, 2021
We are part of a group of clinicians, scientists, and patient advocates who have lodged a formal “Citizen Petition” with the United States Food and Drug Administration (FDA), asking the agency to delay any consideration of a “full approval” of a covid-19 vaccine. The message of our petition is “slow down and get the science right—there is no legitimate reason to hurry to grant a license to a coronavirus vaccine.” We believe the existing evidence base—both pre- and post-authorization—is simply not mature enough at this point to adequately judge whether clinical benefits outweigh the risks in all populations.
The covid-19 vaccines in widespread use have emergency authorizations (EUA), not actual approvals, a crucial regulatory distinction that reflects major differences in the level of regulatory scrutiny and certainty about the risk-benefit balance.
Our petition doesn’t argue that risks outweigh benefits—or that benefits outweigh risks. Rather, we focus on methods and processes, outlining the many remaining unknowns about safety and effectiveness—and suggest the kinds of studies needed to address the open questions.
If the FDA listens to us, they won’t give serious consideration to approving a covid-19 vaccine until 2022. Our first request is that the FDA require manufacturers to submit data from completed Phase III trials—not interim results. Trials by vaccine manufacturers were designed to follow participants for two years, and should be completed before they are evaluated for full approval, even if they are now unblinded and lack placebo groups. These Phase III trials are not simply efficacy studies; they also are necessary and important safety studies (as the study titles say), and all collected data remain invaluable.
We also call on FDA to require a more thorough assessment of spike proteins produced in-situ by the body following vaccination—including studies on their full biodistribution, pharmacokinetics, and tissue-specific toxicities. We ask the FDA to demand manufacturers complete proper biodistribution studies that would be expected of any new drug and request additional studies to better understand the implications of mRNA translation in distant tissues. We call on data demonstrating a thorough investigation of all serious adverse events reported to pharmacovigilance systems, carried out by independent, impartial individuals, and for safety data from individuals receiving more than two vaccine doses, in consideration of plans for future booster shots. We ask the FDA to request necessary studies in specific populations, including those previously infected with SARS-CoV-2, pediatric subjects, and those with immunological or other underlying medical complexities. Given the nature of the novel vaccine platforms, our petition asks for experts in gene therapy to be included among the external committee advising the FDA.
These are several of our major requests. The petition has been signed by a group of 27 clinicians, researchers, and consumer advocates with diverse experiences and thoughts about the pandemic. We all agree that there remain many open, unanswered questions surrounding the efficacy and safety of covid-19 vaccines that must be answered before the FDA gives serious consideration to granting full approval.
These are the reasons why we lodged our petition. There is no need to rush approval to help stop the pandemic because the vaccines already have Emergency Use Authorization. Yet a rushed process is the very possibility that now confronts us. In the past month, Pfizer and Moderna submitted formal applications for “full approval.”
Covid-19 vaccines are already fully accessible to all Americans who want one. EUAs have enabled their widespread use, and can remain in place even after the expiry of the SARS-CoV-2 public health emergency declaration, as is the case for various Zika products. Even without full approval, covid-19 vaccines will remain available for all who want them under EUA.
Some surveys suggest that vaccine hesitancy in the United States is due, in part, to lack of full FDA approval. While approval might lead to increased public confidence in covid-19 vaccines, as well as provide legal support for employer-instituted vaccine mandates, to approve a medical product for these reasons is outside FDA’s regulatory purview. Approval decisions must be driven by the safety and efficacy data. The potential unintended consequences of a rushed approval may contribute to growing mistrust of the US public health and regulatory institutions.
Finally, regarding the elephant in the room: publicly raising any element of hesitation about covid-19 vaccines will be seen by some as irresponsible, stoking unfounded fears in the public’s mind and contributing to the “vaccine hesitancy” problem trumpeted every day. But the alternatives—privately raising concerns or simply remaining silent—are arguably more detrimental to public trust in the long run. Staying silent is not the responsible option. And the implications of only privately raising concerns to regulatory bodies are murky—most would probably not be acted upon, and if they were, it would promulgate the baggage of insufficient accountability and transparency in decision making.
To us, the Citizen Petition seemed the most responsible approach: voice our concerns in our own words, in a professional and transparent manner, through a formal mechanism that can promote accountability in regulatory decision making.
Approving a covid-19 vaccine now risks setting a precedent of lowered standards for future vaccine approvals. The “FDA approved” seal must represent a high bar—and premature licensure of a covid-19 vaccine could seriously damage public confidence in regulatory authorities, particularly if long-term safety issues were to emerge following licensure. Keeping covid-19 vaccines under EUA regulations would also encourage vaccine manufacturers to continue investing resources in completing the necessary safety and efficacy studies for a potential FDA consideration of full licensure in the future.
For each covid-19 vaccine, the benefits may ultimately outweigh the harms. Or not. Or we may end up in a more nuanced position, finding that benefits outweigh harms for some populations, but not others. Only time—and better evidence—will tell. And so it is vital we allow the scientific process the time required to gather and assess the evidence to be confident in the decisions we ultimately have to make.
Our citizen petition and supporting documents are filed under Docket ID FDA-2021-P-0521 on regulations.gov. Anybody can comment on the petition, or read others’ comments, including the FDA’s official reply once it arrives.
—————————-
1
June 1, 2021Electronic SubmissionDivision of Dockets ManagementFood and Drug AdministrationDepartment of Health and Human Services5630 Fishers Lane, Room 1061Rockville, MD 20852CITIZEN PETITIONccine product outweigh the harms for the indicated, recipient population. We are concerned that the premature licensure of a COVID-19 vaccine can seriously undermine public confidence in regulatory authorities, particularly if long-term safety issues were to emerge following licensure.
——————————————————————
Comments to the petition can be made -chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://downloads.regulations.gov/FDA-2021-P-0521-0001/attachment_1.pdf#page=1&zoom=auto,-19,802
I think that when petitions need to be raised however diplomatically in order to protect the health of citizens as well as trust in governments it is hardly surprising that many of us are declining vaccination. But importantly The decision is not just based on contempt for the medical and regulatory bodies which have corrupted trust – but also many of us who are able to access a level of information – and everybody cannot – make as informed judgements about risks versus benefits as much as we possibly can based on what information we , as far as possible find reliable . The push t o get everyone vaccinated is using a tactic of fear and divisiveness – vaccinated good refusniks bad with troubling consequences Hiding the real truth from all but those in the know is treating citizens as pawns in a n. experiment with unknown consequences. Given the truth in formats accessible to all would probably still lead to people consenting to this massive experiment in vaccination Who knows?Secrets and lies make the enterprise an extremely shabby abuse of trust.
A big dose of a reality – Check..
Join David Healy, MD and Angela Peacock, MSW from the Medicating Normal team as they discuss medical groupthink and why the mental health industry does not believe the patients who have been harmed by it. They will also have a frank conversation about the sexual side effects from psychiatric drugs.
https://www.facebook.com/events/210304034256922
Serotonin, Doctors’ Gadgets, Homicide, Sex on a Platter – and more…
It looks like the vaccination of children is going ahead -Parents who decide the rxisks of vaccinating their children are too great are going to find the pressure very hard to resist It is not unreasonable to mistrust what is being told publicly by the MHRA and co.
1
Open Letterfrom UK doctors: Safety and Ethical Concerns SurroundingCOVID-19 Vaccinationin Children to :-
Dr June Raine, Chief Executive, MHRA
We wish to notify you of our grave concerns regarding all proposals to administer COVID-19 vaccines to children.Recently leaked Government documents suggested that a COVID-19 vaccine rollout in children over 12 years old is already planned for September 2021, and the possibility of children as young as 5 years old being vaccinated in the summer in a worst-case scenario.1We have been deeply disturbed to hear several Government and SAGE representatives calling in the media for the COVID-19 vaccine rollout to be “turning to children as fast as we can”.2Teaching materials circulated to London schools contain emotionally loaded questions and inaccuracies3. In addition, there has been disturbing language used by teaching union leaders, implying that coercion of children to accept the COVID-19 vaccines through peer pressure in schools was to be encouraged, despite the fact that coercion to accept a medical treatment is against UK and International Laws and Declarations.4Rhetoric such as this is irresponsible and unethical, and encouragesthe public to demandthe vaccination of minors with a productstill atthe researchstage andabout which no medium-or long-term effects are known, against a disease which presents no material risk to them. A summary of our reasons is given belowand a more detailed fully referenced explanation is available.5Risks and benefits in medical treatmentsVaccines,likeany other medical treatment, come with varied risks and benefits. Therefore, we must consider each product, individually, on its merits, and specificallyforwhich patients or sections of the population is the risk/benefit ratio acceptable.For COVID-19vaccines,the potential benefits are clear for the elderly and vulnerable,however, for children, the balance of benefit and risk would be quite different. We are raising these concerns as part of an informed debate, which is a vital part of the proper, scientific process. We must ensure that there is no repeat of any past tragedies which have occurred especially when vaccines are rushed to market. For example, the swine flu vaccine,Pandemrix,rolled out followingthe pandemic of 2010, resulted inover one thousand cases of narcolepsy,a devastating brain injury, in children andteenagers, before being withdrawn.6Dengvaxia, a new vaccine against Dengue, was also rolled out to children ahead of the full trial outcomes, and 19 children died of possible antibody-dependent enhancement (ADE)before the vaccine was withdrawn.7We must not riska repeat of this with the COVID-19 vaccines, which would not only impact on the children and families affected, but would also have a hugely damaging effect on vaccination uptake in general.No medical intervention should be introduced on a ‘onesize fits all’ basis, but instead should be fully assessed for suitability according to the characteristics of the age cohort and of the individuals concerned, weighing up the risk versus benefit profile for each cohort and the individuals within a group.This approach was outlined last October, by the head of the Government Vaccine Task Force, Kate Bingham, who said “We just need to vaccinate everyone at risk.There’s going to be no vaccination of people under 18. It’s an adult-only vaccine, for people over 50, focusing on health workers and care home workers and the vulnerable.”8
Children do not need vaccination for their own protectionHealthy children are at almost no risk from COVID-19, with risk of death as low as 1 in 2.5 million9.No previously healthy child under the age of 15 died during the pandemic in the UK and admissions to hospital or intensive care are exceedingly rare10with most children having no or very mild symptoms. Although Long-Covid has been cited as a reason for vaccinating children, there is little hard data. It appears less common and much shorter-lived than in adults and none of the vaccine trials have studied this outcome1112. The inflammatory condition, PIMS, was listed as a potential adverse effect in the Oxford AstraZeneca children’s trial13. Naturally acquired immunity will give broader and better lasting immunity than vaccination14.Indeed, many children will already be immune15. Individual children at very high risk can already receive vaccination on compassionate grounds16.Children do not need vaccination to support herd immunityAlready, two thirds of the adult population have received at least one dose of a COVID-19 vaccine17. Models that assume vaccination of children is required to reach herd immunity have failed to account for the proportion who had immunity prior to March 2020 and those who have acquired it naturally18. Recent modelling suggested that the UK had achieved the requiredherd immunity threshold on 12 April 202119.Childrendo not transmit SARS-CoV-2 as readily as adults, moreover adults living or working with young children are at lower risk of severe COVID-1920. Schools have not been shown to be the focus on spread to the community, teachers have a lower risk of COVID -19 than other working age adults21. Short-term safety concernsAs of 13thMay, the MHRA22has received a total of224,544adverse events, including 1,145deathsin association with SARS-CoV-2 vaccines. Reports of strokes due to cerebral venous thromboses were initially in low numbers but as awareness increased,many more reports led to the conclusion that AstraZeneca vaccine should not be used for adults under 40 years of age and this unpredicted finding has also led to the suspension of the Oxford AstraZeneca children’s trial.Similar events have been noted with Pfizer & Moderna vaccines on the US adverse reporting system (VAERS)23and it is likely that this is a class effect related to production of spike protein. New UK guidelines on managing Vaccine-Induced Thrombotic Thrombocytopenia (VITT)24include all COVID-19 vaccines in their advice. The possibility of further unexpected safety issues cannot be ruled out. In Israel, where the vaccines have been widely rolled out to young people and teenagers, the Pfizer vaccine has been linked to several cases of myocarditis in young men25and concerns have been raised about reports of altered menstrual cycles and abnormal bleeding in young women following the vaccine.26Most concerning with regard to possible vaccination of children, is that there have now beena number of deaths associated with vaccination reported to theVAERS system in theUS, despite the vaccines only being given to children within trials and a very recent rollout to 16-17 year olds27. Long-term safety concernsAll Phase 3 COVID-19 vaccine trials are ongoing and not due to conclude until late 2022/early 2023. The vaccines are, therefore, currently experimental with only limited
short-term and no long-term adult safety data available. In addition, many are using a completely new mRNA vaccine technology, which has never previously been approved for use in humans28.The mRNA is effectively a pro-drug and itisnot known how much spike protein any individual will produce. Potential late-onset effects can take months or years to become apparent.The limited children’s trials undertaken to date are totally underpowered to rule out uncommon but severe side effects.Children have a lifetime ahead of them, and their immunological and neurological systems are still in development, making them potentially more vulnerable to adverse effects than adults. A number of specificconcerns have been raised already, including autoimmune disease and possible effects on placentation and fertility.29A recently published paper raised the possibility that mRNA COVID-19 vaccines could trigger prion-based, neurodegenerative disease30.Allpotentialrisks, known and unknown, must be balanced against risks of COVID-19 itself, so a very different benefit/risk balance will apply tochildren than toadults.ConclusionThere is important wisdom in the Hippocratic Oath which states, “First do no harm”. All medical interventions carry a risk of harm, so we have a duty to act with caution and proportionality. This is particularly the case when considering mass interventionin a healthy population, in which situation there must be firm evidence of benefits far greater than harms. The current, available evidence clearly shows that the risk versus benefit calculation does NOT support administering rushed and experimental COVID-19 vaccines to children, who have virtually no risk from COVID-19, yet face known and unknown risks from the vaccines.The Declaration of the Rights of the Child states that, “the child, by reason of his physical and mental immaturity, needs special safeguards and care, including appropriate legal protection”.31As adults we have a duty of care to protect children from unnecessary and foreseeable harm.We conclude that it is irresponsible,unethicaland indeed,unnecessary, to include children under 18 years in the national COVID-19 vaccine rollout. Clinical trialsin children also pose huge ethical dilemmas, in light of the lack of potential benefit to trial participants and the unknown risks. The end of the current Phase 3 trials should be awaited as well as several years of safety data in adults, to rule out, or quantify, all potential adverse effects.We call upon ourgovernmentsand the regulatorsnot to repeat mistakes from history, and to reject the calls to vaccinate children against COVID-19. Extreme caution has been exercised over many aspects of the pandemic, but surely now is the most important time to exercise true caution-we must not be the generation of adults that, through unnecessary haste and fear, risksthe health of children.Signatories…too many to list see on line
GPs will need to risk assess mass patient data extraction, says ICO
mass patient data extraction
Awil Mohamoud
11 June 2021
Exclusive Each GP practice will need to perform a data protection impact assessment (DPIA) before NHS Digital’s controversial mass extraction of patient data from practice systems takes place, Pulse has learned.
The Information Commissioner’s Office (ICO) has told Pulse that General Practice Data for Planning and Research (GPDPR), ‘as it involves processing health information (special category data)’, is ‘likely to result in a high risk to individuals’.
This means data controllers – GPs, in this case – ‘will need to perform a DPIA’, it said.
The ICO explained that this is a legal requirement since the new Data Protection Act came into force in May 2018 and comes as Pulse revealed last month that privacy campaigners fear the new automatic extractions of data will be ‘far bigger’ and ‘more intrusive’ than the scrapped care.data project.
An ICO spokesperson said that the DPIA is ‘a way to help you identify and minimise data protection risks’.
It further added that if any high risks are identified which ‘cannot be minimised through additional measures’, controllers ‘must consult the ICO’. Such a process could take 8-14 weeks and may result in the ICO blocking the data processing from taking place.
However, NHS Digital told Pulse that it has prepared and shared its own DPIA with the ICO and that this covers the risks from both the perspective of the GP and NHS Digital.
In addition, NHS Digital intends to make a GP DPIA available for practices to use ‘if they wish’, to ‘support them to consider the risks and be confident they have discharged their obligations under the Data Protection Act 2018 and UK GDPR’.
It also stressed that with regards to the GPDPR data collection, GP practices ‘are legally obliged to share the data requested with NHS Digital under the Health and Social Care Act 2012’.
But Phil Booth, coordinator of data confidentiality advocacy group MedConfidential, argued that general practice needs to design its own DPIA.
He said: ‘Clearly, NHS Digital has designed and is running the system. It must publish its DPIA, as [it knows] what the system is and only [NHS Digital] can describe it.
‘But, they are clearly conflicted. They are doing a DPIA of their own system, which is their duty, but, in doing so, they may miss or not think of certain aspects. They certainly won’t be thinking of it from the perspective of the GP, as a data controller.
‘There clearly needs to be a DPIA that is independent of the NHS Digital one, which is owned and backed by the RCGP, BMA and maybe LMCs.’
Hampshire GP and data autonomy advocate Dr Neil Bhatia further stressed that GP concerns ‘would need to be referred to ICO, as there are high risks of data subject rights not being upheld, [including] the right to be informed’.
But he suggested particular groups of GP practices should team up to consult the ICO.
He told Pulse: ‘The ICO won’t be very happy if 7,000 GP practices do it. One might hope that a representative sample of practices [consult the ICO] – rural practices, university practices, those with a very high number of elderly patients who are not necessarily digitally enabled.’
De Montfort University professor of cyber security Professor Eerke Boiten told Pulse that NHS Digital providing a DPIA for individual practices to use will ‘save GPs the individual effort’ of filling in the forms themselves but said those with additional ‘insight or worries’ could decide to design their own.
He said, however, that GPs can ‘reasonably justify’ allowing the data to be processed as NHS Digital ‘is legally required to be a responsible holder of central medical data’.
An ICO spokesperson said: ‘People having confidence in how their data is being used and shared is an important part of people’s broader trust in an organisation.
‘When handling health data, organisations need to take extra care and put safeguards in place to protect people’s privacy, ensuring their data is not used or shared in ways they wouldn’t expect. We have recently produced updated guidance and tools to help organisations share data safely and with confidence.
‘We are aware of the GP Data for Planning and Research programme and we’ve discussed with NHS Digital their data protection obligations.’
It comes as the Government this week delayed the date for the GPDPR extraction from 1 July to 1 September, amid concerns the public has not been sufficiently informed.
Health secretary Matt Hancock also said that the deadline for patients to opt out of the data extraction would be extended from the previous date of 23 June, but he has not yet announced a new date.
This followed concerns raised by the BMA and RCGP, which have both advised on the GPDPR system that will replace the GP Data Extraction Service (GPES).
Following the announcement, Mr Hancock asked former RCGP chair Professor Helen Stokes-Lampard to advise the Government on the project.