This talk was given at the Annual Meeting of the International Society for Ethical Psychology and Psychiatry on October 28. The Video version of HealthCare Gone Mad is linked here. The slides and text are below. There will soon be an official ISEPP link to the talk and a Q and A, which will be added here as soon as its available.
Since giving the talk, I’ve discovered that a very early version of this talk was given in February 2021 – See Sex and Evidence Based Medicine and I Can’t Breathe. I can’t Breathe picks up on George Floyd’s now famous phrase and combines it with white folk feeling they can’t breathe for other reasons.
Slide 1: Everyone can likely see 2 Faces and a Vase here. The word RANDOM can also be seen two ways. Random behavior was seen as key sign of madness. Now it is seen as the path to Wisdom and Truth.
In the 1960s, phrases like Do not adjust your TV, the World has gone Mad were popular. In the 2020s we need something more like Do not adjust your Reality, the Virtual World has gone Mad.

Slide 2: You might see 3 ordinary guys here or 3 healthcare experts.
The ordinary guys have Post SSRI Sexual Dysfunction – PSSD – a state of sexual wipe-out after you stop an SSRI, which can last forever. In talking about their PSSD on a Podcast, it becomes clear these 3 guys have an extraordinary expertise on what is wrong with healthcare today. Here are some quotes.
Bryn: Getting PSSD undermines your faith in the medical establishment and the whole system of science – the fact that none of these professionals understand this condition or know anything about it when we know we have it – it’s not some vague feeling. Losing you libido is equivalent to going blind or deaf, it’s that level of sensory impairment
Roy: The same can be said for losing your emotions – I felt I lost two senses – my sexuality and my emotions
Simon:
Bryn: If we want to be believed we have to remember just how incredible our story sounds… It’s a hard thing to wrap your head around… My Dad says it’s not possible a drug could cause these effects, it wouldn’t be on the market
Slide 3: Let me translate. Both Bryn and Roy say something like: Doc the hand growing out of my shoulder and my missing arm is because my mother took a pill while she was pregnant with me. the doctors says: don’t be silly your arms look just fine to me. A great pill like thalidomide couldn’t cause a problem like this.
Having a child born with the problems caused by Thalidomide had a huge impact on lots of families as any abuse can. Simon outlines the impact of his PSSD on his family in an extraordinary fashion. What he outlines will be gripping listening for anyone interested in issues like this.
Bryn then introduces his Dad saying that ‘They’ wouldn’t let a drug on the market that caused your hand to grow out of your shoulder or PSSD.
Slide 4: Wonder Drug is about Thalidomide in America. The usual narrative is Americans were saved from the worst but Wonder Drug makes clear this drug was handed out to thousands of American doctors who gave it to tens of thousands of women and there were hundreds of babies born with defects, which FDA went out of their way not to find. What FDA did next caused Roy, Bryn and Simon’s PSSD problem and kills and maims tens of thousands of Americans each year. My talk is about this US contribution to global drug induced deaths and injuries.
Slide 5: Primed by the last slide you may be looking for this child’s birth defects. Its her mother who has the birth defects. Those injured by drugs have thought us a lot about Being Able and shone a light on the way forward in healthcare and life for all us.
Slide 6: Thalidomide had been made in France and tested for tuberculosis, when in 1948 Tony Hill ran the first randomized controlled trial (RCT) – of streptomycin for tuberculosis. Hill used randomization as a method of fair allocation – nothing here about the randomized mysticism you hear about today. This RCT found out less about streptomycin than a prior non-randomized trial in the Mayo Clinic, which showed it can cause deafness and tolerance develops rapidly.
Slide 7: Here is Hill in 1965 taking stock of RCTs. If randomization, double-blinds or placebo got in the way of a doctor evaluating a drug, he said, get rid of them. Hill believed in Evident Based rather than Evidence Based Medicine.
He said RCTs were needed in 1950 to work out if anything worked – a way to eliminate ineffective and unsafe drugs. By 1965 we had lots of drugs that worked – none discovered through RCTs. He never envisaged companies using RCTs to get weaker drugs on the market.
All drugs do a thousand things. An RCT focusses on one which might be useful for treatment. purposes. In focusing on one element, Hill is saying RCTs are not a good way to evaluate a drug. All RCTs generate ignorance. But we can bring good out of ignorance if we remain on top of what we are doing.
RCTs, he said, produce average effects which don’t tell a doctor how to treat the person in front of them. Hill never saw RCTs replacing clinical judgement. If we let that happen we’d have gone mad.
Slide 8: Louis Lasagna was the leading proponent of RCTs in the US. In 1956 he suggested in addition to FDA asking companies to prove their drug is safe, it should also require companies to show their drugs worked. RCTs he figured were a new quick and cheap way to do this. If a drug doesn’t work it can’t be safe.
In 1960, Lasagna ran an RCT which showed thalidomide was an effective sleeping pill. The trial missed the SSRI like agitation, suicidality and sexual dysfunction it causes.
Slide 9: In the Oval Office 61 years ago, John Kennedy signed a new Food and Drugs Act and hands the pen to Frances Kelsey who blocked thalidomide’s approval in the US.
The 1950s was a miraculous decade for new drug discovery – psychotropics, antihypertensives, antibiotics, hypoglycemics, steroids – all without an RCT. Drugs that cured problems getting in the way of us living the lives we wanted to live – unlike today’s drugs.
Kelsey averted a Thalidomide disaster using a 1938 Act, which like regulations for food, cars, stock markets – focused on safety. But something had to be seen to be done about thalidomide.
The new 1962 Act adopted Lasagna’s proposal and required companies to prove their drugs were Effective and Safe. For most people the word effective means ‘works’ which means saves lives or reduces disability. Almost no drugs since 1962 have been shown in company trials to save lives or prevent disability. To show lives being saved would need trials lasting a decade. And, the hurdle to stop thalidomide happening again was one it sailed over in an RCT. The 1962 Act would have licensed it.
Frances Kelsey was the only woman in the room when this Act was signed. The Act replaced her professional judgement, that a sleeping pill, even if effective, should not cause a peripheral neuropathy, with an algorithm that biased development toward efficacy. It wasn’t clear then that company trials would replace clinical judgment – which Hill and Lasagna thought was critical – with algorithms. Designed to contain pharmaceutical companies, the New Act handed control over to companies.
Slide 10: Lasagna made a bad mistake, but with good intentions. RCTs were a poorly understood technique in 1962. Here in the 1990s you have him admitting to a mistake:
In contrast to my role in the 1950s which was trying to convince people to do controlled trials, now I find myself telling people that it’s not the only way to truth.
Evidence Based Medicine has become synonymous with RCTs even though such trials invariably fail to tell the physician what he or she wants to know which is, which drug is best for Mr Jones or Ms Smith – not what happens to a non-existent average person.
Slide 11: Like Vase and Faces you may see both an Older and a Younger Woman here.

Slide 12: The image shows Emer Cooke, CEO of EMA and Albert Bourla CEO of Pfizer.
As with all efforts to regulate pharmaceuticals, the 1962 legislators claimed they did not want to interfere with the practice of medicine. In 1914 they made heroin and cocaine prescription only and extended this to all drugs in 1951 – giving doctors a police function that doesn’t sit well with healing or professionalism.
We think we’re the consumers of drugs. We aren’t. Doctors consume drugs by putting them in our mouth – a situation ripe with a potential for abuse – especially as Pharma figure few doctors have any thoughts in their heads not put there by us or our competitors.
RCTs steered prescription only drug consumption toward diseases. Pharma’s answer was to give us multiple diseases. Are doctors going to stop this? Not when the greatest concentration of marketing power on earth only has to focus on comparatively few consumers
Slide 13: Here is Hill in the same 1965 lecture about Controlled Trials saying:
Clinical Trials have invaded the sales talk: “A double-blind cross-over trial on 22 patients has shown XYZ is the antibiotic of choice.”
What would Drs have made of that 20 years ago? It would not have been a selling point. But clearly it is thought to be so today”.
Like RCTs then – Pharma are the cheerleaders for EBM, which doctors think contains pharma.
Slide 14: At the risk of offence, the Catholic doctrine of Transubstantiation claims the wafer you see here isn’t a wafer – it and the wine are the actual Body and Blood of Christ. It takes the eye of faith for you to see this. I am not saying believers are wrong to see things this way.
The word randomization now has similar religious connotations. Even if done by the company equivalent of a pedophile priest, the evaluation of drugs is mystically transformed – poisons out of which we hope to bring good become sacraments – from which no harm can come. I am saying RCT believers are wrong.
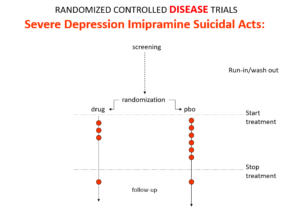
Slide 15: The 1950s gave us the best antihypertensives, antibiotics and psychotropics ever – all without RCT input. Imipramine, the first antidepressant, is stronger than SSRIs. It treats melancholia which SSRIs can’t. Melancholia increases the risk of suicide 80-fold.
Imipramine was launched in 1958. By 1959, while praising it to the skies, doctors practicing Evident Based Medicine could still see it made some people suicidal. Stop the drug the suicidality clears. Re-introduce it, suicidality comes back.
Because it treats this high risk condition, an imipramine v placebo RCT in melancholia should show more red dot suicide attempts on placebo even though imipramine can cause suicide. This would look like evidence imipramine cannot cause suicide.
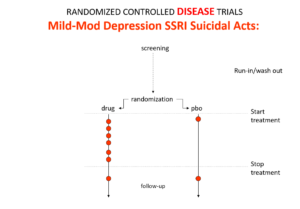
Slide 16: In the mild depression trials that brought SSRIs to market – there is an increase of suicidal events compared to placebo in people at little or no risk of suicide.
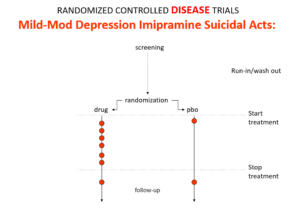
Slide 17: An imipramine comparator in the same trials also caused suicides. Context means the answer RCTs can give on the same drug can be quite opposite. There is no absolute truth.
We get opposite answers because these are Treatment Trials not Drug Trials. Believers claim RCTs control all confounders in all known universes, but if a condition and treatment produce superficially similar effects, RCTs cause confounding that RCTs cannot solve.
If a patient has a problem on a drug in a trial, as Hill said, a doctor needs to work out what is happening but in RCTs now clinicians are not supposed to use their judgment.
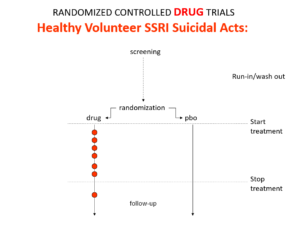
Slide 18: Healthy volunteer studies are Drug Trials. In Drug Trials in the 1980s, companies found SSRIs cause volunteers to become suicidal, dependent and sexually dysfunctional. We heard nothing about this when the drugs launched because Drug Trials enabled companies to engineer Treatment Trials to hide these problems. See God Does not Roll Dice for more on this.
Slide 19: In 1998, Don Schell, a tough oilman from Wyoming with a minor sleep problem was put on Paxil. 48 hours later he shot his wife, his daughter, his grand-daughter and himself. His son-in-law took a lawsuit against GlaxoSmithKline (GSK) – Tobin v SmithKline.
In the Tobin case, Ian Hudson, Chief Safety Officer of GSK was asked – Can SSRIs cause suicide. He says GSK practice Evidence Based Medicine (EBM) and base their views on RCTs, which do not show the Paxil sacrament has any side effects – it does not cause suicide or homicide.
Even the Catholic Church recognize the Eucharistic can have side effects and minimize the gluten in it, but these down here on earth considerations don’t bother GSK.
A jury of 12 Wyoming folk with no background in health thought Hudson was Mad. They dismissed EBM in favor of Evident Based Medicine. It was obvious Paxil caused these homicides, they said, and GSK were guilty of negligence.
Hudson, however, later became the CEO of Britain’s drugs regulator, and these Mad views are dug in at the top of FDA, EMA, and WHO.
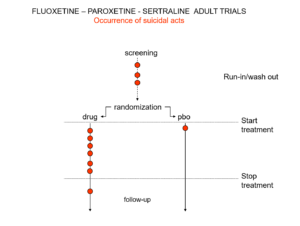
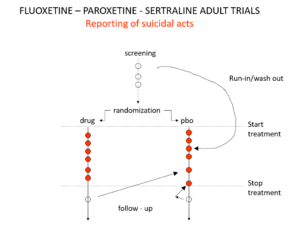
Slide 20: Hudson knew something else the jury were not told. Here are the suicidal events in the trials that brought us Prozac, Paxil and Zoloft around 1990. Note the events under screening – a 2 week pre-trial washout period when people are taken off prior drugs before starting the new treatment or placebo. Washing out psychotropic drug can push people into withdrawal and suicide.
Slide 21: When submitting the data to FDA, companies moved events as you see here. They argued that people in washout were not on active treatment – which is the same as being on placebo. The same argument was used for end of trial maneuvers. FDA turned a blind eye to these maneuvers.
Slide 22: In the mid-1980s, Paul Leber head of FDA’s Psychopharm Division said ‘For 20 years we have let companies run trials comparing a new to an old drug – these can’t tell us if a drug works. Both drugs might look the same – but neither may be working. You have to beat placebo for an assay to work. This caused a crisis because SSRIs couldn’t beat placebo.
Slide 23: Zoloft Approval Committee Meeting 1991
Leber:
How do we interpret… two positive results in the context of several studies that fail to demonstrate that effect? I am not sure I have an answer, but I am not sure the law requires me to have an answer.
In a sense, the sponsor could just do studies until the cows come home until he gets two of them that are statistically significant by chance, walks them out and says he has met the criteria.
What this shows you is FDA don’t do science – they are bureaucrats ticking boxes and the box is are there 2 assays that meet a standard FDA and the companies have agreed between them. See Let Them Eat Prozac.
Slide 24: There are more dead bodies on SSRIs than on placebo in RCTs yet Ian Hudson told you the drugs work. Working is based on surrogates for lives saved – lipid levels rather than heart attacks. For antidepressants it’s the Hamilton Rating Scale for Depression.
Health service managers see a scientific interview here – better than you being asked about your holidays, a recipe, last night’s Republican Party debate. What Hamilton saw was a checklist that standardized interviews:
It may be we are witnessing a change as revolutionary as the introduction of standardization and mass production in manufacture. Both have their positive and negative sides
Checklists produce standardized but possibly disastrous interviews. The Hamilton scale has a suicide item. Suicidality can stem from the illness or the drug. If caused by the drug you should rate 0. If the illness you might rate 3 or 4. Depression and antidepressants affect sleep, sex, anxiety and depression – a scientific interview requires judgement calls at these points. It can’t be mindless. If the drug is causing problems and raising your score, and you score 4 and increase the dose, you may kill the patient.
Regulators run assay systems – they are looking at the effect of a drug on an assay system with the outcome measure being an average rating scale score. This average contains people doing well and people doing poorly – it does not inform clinical practice,
In the 1980s, we brought problems to doctors seeking help to live the lives we wanted to live. Now, rating scales, sometimes left in the waiting room, ensure doctors produce figures for which putting you on rather than taking you off a company drug will seem a good option. Doctors now help people to live the life Pfizer wants them to live. This is no longer medicine.
Slide 25: When approving drugs, regulators assess assays. Companies design Randomized Controlled Assays to get a product on the market, not RCTs to inform clinical care.
Covid RAT tests are examples of an assay. The cycle threshold can be set so that a drop of pond-water can have Covid or only someone dying from Covid in ITU will have it. We set an arbitrary standard and say meet this and you have to stay at home.
In the same way regulators and companies agree on an arbitrary standard – meet this and you can use the word antidepressant even though more people die on the drug.
Slide 26: You may see a Duck here, a Rabbit or Both. It can be hard to tell Randomized Controlled Assays and Randomized Controlled Trials apart but have very different consequences.
Slide 27: Fifty years ago, Britain joined the EU and ran into trouble. They were told the nation’s favorite chocolate could not be called chocolate. It didn’t have the right quota of cocoa solids. This Deep State fuss led to Brexit.
FDA regulate Food and Drugs. Faced with butter, chocolate or drugs, it requires companies to meet an assay standard – so much cocoa solids, animal fats, or points on a Rating Scale in 2 trials. Meet that and FDA let you use the words chocolate, butter, or antidepressant. FDA do not decide if this is good butter, or if chocolate is good for you, or police the medical articles claiming the drug works.
Slide 28: Laxatives bring out an important point about regulation. Almost no drugs are Magic Bullets. They offer Therapeutic Principles. In the case of laxatives there are 4 therapeutic principles – 4 different ways to work and only one of these will likely help your constipation. Get the wrong one and you may end up on 4 laxatives that do different things, perhaps causing treatment resistant constipation.
The same is true for antihypertensives, hypoglycemics, and almost all drugs. If FDA license Drug X as an antihypertensive and if you’ve got mildly raised blood pressure, a doctor will give X without thinking. If there is no improvement on W, X and Y but a benefit from Z, s/he will leave you on all 4 for your treatment resistant hypertension because they are all antihypertensive.
Doctors used to be good at working out which therapeutic principle was right for you. But companies just want you to use their laxative for all constipations and once licensed to use the word laxative they work to replace clinical skills with a reflex prescribing of their laxative. This is bad for you, good for companies. It has helped create a polypharmacy pandemic
Slide 29: Antidepressants also have 4 therapeutic principles. A serenic effect with an SSRI, a drive enhancing effect on norepinephrine reuptake inhibitors, a tonic – better sleep and appetite – effect on mirtazapine like drugs and a treatment for melancholia in the case of Tricyclics, perhaps because of the euphoriant effect linked to their anticholinergic action.
RCAs make all these look the same – the same rough score on a scale. Its like putting humans, cows and dogs through a mincer – what comes out the far side all looks the same.
When regulators let companies use the word antidepressant it makes them all seem like Magic Bullets rather than Therapeutic Principles. Company marketing stresses Magic Bullets which hands the narrative to them – all doctors need to do is prescribe and all we need to do is take.
Therapeutic Principles take the narrative away from marketing departments – we and our doctors have much more say over which drug might be the best bet for us.
With depression, the consequences are grim. Until recently there was no such thing as treatment resistant depression. Now there is. It stems from doctors just chucking pills at people. Psychotropic drugs can give healthy volunteers PSSD or a dysthymic mood, making them suicidal with disorders that have no treatment. From Canada to Belgium, young people in these states are now being offered MAiD – medical assistance in dying.
Slide 30: FDA approval does something else. After thalidomide women avoided drugs in pregnancy and now avoid soft cheeses, processed meats, alcohol, coffee and hot showers. But soft cheeses do not come with a label on them saying Eat This. Labels like this, as in Alice in Wonderland, can shape behavior with crazy consequences.
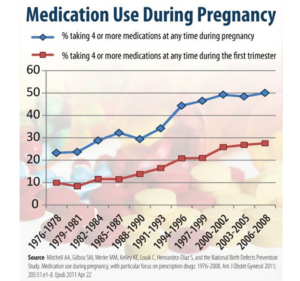
Slide 31: This graph which only takes us to 2008 – things are worse now – show you that women are consuming more and more drugs in pregnancy with antidepressants being the most commonly taken drugs aside from antibiotics. We have signs in restaurants saying don’t drink if you’re pregnant but nothing about SSRIs which are linked to a 10-fold increase in Fetal Alcohol Syndrome.
Its WEIRD — White Educated Influential Rich Dames are leading the way – not just to taking meds in pregnancy but to getting involved in RCTs in pregnancy.
Slide 32: On the left is the most famous assay in medicine – Study 329. An example of the assays companies do to get a drug on the market – Paxil for children in this case. The journal has the highest impact factor in children’s mental health. The authorship line is to die for – except the author is not there. Sally Laden a medical ghostwriter wrote it. This ghostwriting holds true for almost all articles on any on-patent drug in any area of medicine. See Children of the Cure.
This published assay reports that Paroxetine worked well and was entirely safe.
On the right is the only article reporting the results of a company assay – Study 329.org – that has had access to the company data that I know of. Our restoration shows that when you have the data, the assay claim that the drug works wasn’t right and the claim it is safe isn’t right – we found 1 in 6 children on Paxil had a significant behavioral event – mostly suicidality. Using Ian Hudson’s rules for cause and effect – Paxil caused suicidality in these children.
Slide 33: FDA don’t get the assay data. In Study 329, FDA knew nothing about a 15 year old, who 2 weeks after going on Paxil ends up on the street waving a gun around threatening to kill people. Nothing in the company account of the study that FDA reviewed hinted at this. GSK found a loophole that let them conceal the details of children like this from FDA. You need his name and contact details to find out what happened and FDA don’t have this. In Study 329, 4 children disappeared through this loophole – all of them on Paxil.
Slide 34: A year after Study 329 was published, GSK applied to have Paxil approved for depressed teenagers. Here is part of a letter FDA sent GSK approving Paxil in pediatric depression that says GSK have told them Study 329 is negative. FDA agree – all 3 trials are negative. FDA will still approve Paxil for kids. FDA also agree with GSK’s suggestion it would be better not to mention the negative trials in the label of the drug. Why would FDA agree to this?
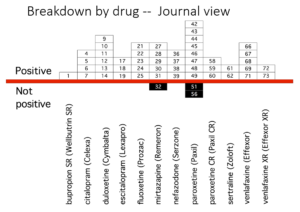
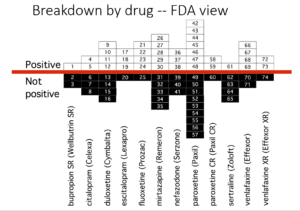
Slide 35: This slide from Erick Turner’s 2008 article shows that almost all published adult ‘trials’ on the antidepressants we know and use indicate the drugs work well and are safe.
Slide 36: This slide shows the FDA view of these trials. Many published as positive were negative to add to the unpublished negative trials. Over 50% of antidepressant trials are negative. Meta-analyses now pitch sertraline as the drug to take – this slide suggests it’s one you should avoid.
You need to remember looking at this – it’s not FDA’s job to police the medical literature. Whose job is it? Doctors and journal editors. I should also tell you Prozac didn’t work in the pediatric trials either.
Slide 37: Audrey Bahrick, a Senior U Iowa Psychologist, was the first person to publish on PSSD. In 2008, she approached her Senator Chuck Grassley who was very concerned about FDA’s record on safety. Grassley helped bring about Black Box Suicide Warnings. She told him about this new hazard. He wrote to FDA. Here is his note to her with FDAs response.
Slide 38: Stephen Mason FDA Assistant Commissioner responds:
It is not possible for FDA in any individual case to determine if the discontinued SSRI, the underlying disorder, or some other unknown factor is responsible for causing sexual dysfunction.
Slide 39: In 2018, a bunch of us, including Audrey, Petitioned FDA to include PSSD in SSRI labels. We also petitioned EMA. Both FDA and EMA were offered reports from over 80 named people with PSSD and over 30 doctors reporting they could see no explanation other than the drug for the problem. FDA and EMA were told the reports were aimed at enabling them to contact people and work out if the drug had caused the problem. There was no response from FDA.
EMA said – please send. I asked a week later if the package had arrived and was told yes and as per procedure they had taken care on arrival to remove all names.
We can work out if FDA’s inability to establish if a drug causes a problem is down to a lack of competence, a lack of motivation, or if some other factor is responsible for FDA’s inability.
If doctors can’t work out if a drug has caused a problem, medical practice would become impossibly dangerous. How do they do it? If Stephen Mason had called Audrey or me he could have been told details like the ones I heard from the first person I met who had PSSD who told me she could take a hard bristled brush and rub it up and down her genitals and feel nothing.
There is no psychiatric disorder that can cause this. Details like this would have helped FDA move things forward. So when FDA eliminate your name and contact details – they lose the possibility to work out what is happening. Your name counts.
Second FDA had just put all their eggs in a Big Data basket – analyzing all the signals from prescriptions and medical records was going to tell them what caused what – but a decade later this approach has found nothing. This is boys with gadgets – it’s not science. PSSD coming on after stopping a drug is also the kind of problem that Big Data can’t cope with.
Slide 40: The antidepressant link to sex goes back to 1960. The first antidepressants were more powerful drugs than the SSRIs. They cure melancholia – a severe mood disorder SSRIs cannot help. People with melancholia lose interest in everything, including sex. But doctors 60 years ago could distinguish a melancholia induced from a drug induced sex problem.
SSRIs are for mild depressions, where there is no loss of libido, but we get told by doctors now our new sexual difficulties are caused by our depression not our pills.
By 1970, we knew one tenth of the dose of older antidepressant could cause genital numbness within 30 minutes which could be used to treat premature ejaculation.
By 1980, we knew that stopping treatment could cause an enduring sexual dysfunction.
In company healthy volunteer trials of SSRIs most volunteers complained bitterly of sexual dysfunction. In later clinical trials, doctors like me were told not to ask about sex. This left companies able to claim sexual dysfunction affected less than 5% of people. See Antidepressants and Sex History.

Slide 41: One of these two is or was on an antidepressant and unable to make love. Like SSRIs and Homicide, the sexual effect of SSRIs impacts significantly on people not on a drug.
- How come we’ve known this for decades, but no-one put on these pills gets told about this hazard?
- If we tell a doctor about PSSD, why are we laughed at and risk being detained for crazy ideas?
- If not us. who are doctors listening to?

Slide 42: NHS digital here reflect regulators EMA and FDA – and all guideline makers.
They say they list the most common side effects, but there are no sexual side effects here, which are by far the most common. When you ask NHS why – they say we do not want to deter people from taking their drugs. In doing so, regulators and guideline makers are getting us to live the life companies want us to live rather than helping us to use company products to live the lives we want to live.

Slide 43: NHS Digital hint at sperm counts falling – in fact they plummet. Women are not told SSRIs double the rate of miscarriages, voluntary terminations, and birth defects. I’ve contacted Pro-Life folk like the Pope who you’d imagine would want to ask questions about this but nothing doing. Something here seems hypocritical.
Slide 44: In May 2019 a BMJ feature claimed Brits are not making love. It blamed depression not the meds. BMJ’s lawyers would have blocked publication if it fingered medication. With 15% of the Western world on them, at least 20% of folk are not making love the way they might wish and maybe up to 50% in some areas.

Slide 45: A year later, the UK fertility rate for 2020 of 1.56 – way below the 2.0 replacement rate. Part of this is just an aging population. But if one fifth of us are not making love, part must be SSRI related.
I’m Irish and with millions of other Irish have hugely improved the UK. I think immigration is a good thing. One third of births in the UK noted above were to mothers born outside the UK. Two-thirds of births in London are to a parent born outside the UK.
The native population takes far more SSRIs than immigrant communities. The fertility rate of the native population may be no more than 1.0. In this case, these drugs don’t only affect the person on them and the person you share a bed with – they affect the entire country.
The problem is not just the chemicals. Medicines are chemicals plus information. The problem is an often fraudulent medical literature and lack of access to data from company assays. These assays are designed to get drugs on the market and get you to live the life companies want you to live. They are not designed to inform clinical practice, or help either us individually or us as a country to live the life we want to live. We call this Evidence Based Medicine.
Slide 46: This is one of the most important political matters today issue. It should but won’t feature in presidential debates. Do we vote Left or Right. Keep looking at this Lady and you will see her rotate left and rotate right.
Slide 47: By 1983, a view was gathering pace that RCTs offered the scientific way to establish if a drug causes an adverse event.
Spontaneous reporting is “the least sophisticated and scientifically rigorous . . . method of detecting new adverse drug reactions” – Rossi et al 1983
In response Louis Lasagna, perhaps chastened by his experience of doing an RCT on thalidomide and finding it had no side effects at all said:
This may be true in the dictionary sense of sophisticated meaning ‘adulterated’ . . . but I submit spontaneous reporting is more ‘worldly-wise, knowing, subtle and intellectually appealing’ than grandiose, expensive RCTs. – Lasagna 1983
Slide 48: Seven years after Lasagna said that good clinical interviews were the way to recognize a drug was causing a problem, 3 Boston clinicians claimed fluoxetine caused 6 people to become suicidal. Analyzing the cases closely using standard scientific approaches to link drug causes to adverse effects, this article established that fluoxetine could make some people suicidal.
Lots of other groups reported similar findings – cases of people who were challenged, dechallenged and rechallenged with an SSRI. There was no way to explain what happened except that fluoxetine can cause some people to become suicidal. This was/is Evident Based Medicine.
Slide 49: In response, BMJ published an article in which Eli Lilly, the makers of Prozac, claimed an analysis of their clinical trials gave no evidence Prozac made anyone suicidal. The cases being reported were sad, they said, but anecdotal – and the plural of anecdote is not data. Depression was the problem not fluoxetine. Clinical trials are the science of cause and effect. Doctors, the public, media, and politicians were being asked – are you going to believe the science or the anecdotes?
This article created Evidence Based Medicine and just as with RCTs 30 years earlier, the people most commonly exhorting doctors to practice EBM today are Pharma companies.
In fact, the original phrase is the plural of anecdotes is data – otherwise Google wouldn’t work. Twenty years earlier the Pedophile Liberation Front were using Lilly’s argument.
The idea the disease is responsible for suicide attempts and suicides in healthy volunteers is hard to believe but companies can wheel out experts to say just that.
But the key point is Evident Based Medicine is the science. In so far as it contains company trials, Evidence Based Medicine is an artefact. My challenge to you is which are you going to believe the Science or the Artefact?
The Science of Medicine lies in making hard judgement calls. The made by algorithm approach, combined with inappropriate statistics, creates artefacts not science.
Among other things, in this very article there was an excess of suicidal events on Prozac compared to placebo, as you’ve seen, and Lilly used inappropriate statistics to argue this away.
Lilly also cooked the books as you’ve seen earlier . When you get their trial data, and undo the manipulations, the Teicher Evident Based Medicine and Lilly Evidence Based Medicine approaches here can be reconciled – as you might expect with real science.
But let’s says there had been a real incompatibility. As you’ve seen the first antidepressants that treated melancholia cure an illness that causes suicide, but they could also cause suicide. So in an RCT of these older drugs in melancholia we would expect these older drugs to save more lives than they take – but in mild depression it would be the other way around.
Resolving discrepancies is how we do science. But Lilly are not in the business of being scientific – resolving discrepant observations, recognizing that both Evident and Evidence Based Medicine might be right. Lilly’s argument is a religious one – a dogmatic one – they forbid us to believe the evidence of our own senses.
Slide 50: This is what has led to this situation today – if you have a side effect on a drug, you face a Tiananmen Square moment. You will be on your own against a ghostwritten academic literature, with no access to the data from studies and up against regulators who have not seen the data but who even knowing negative trials of your drug are published in good journals saying the drug worked well and is safe do not say anything.
These articles are built into guidelines that dictate what your doctor prescribes and his inability to recognize your problems when you bring them to him.
You will be on your own. Your doctor won’t be there beside you.
Slide 51: What happens your doctor when you report a problem. When pilots report a problem, safety systems pay heed – they know she won’t fly if they don’t because if you die, she dies.
Jane Frazer on the right is the CEO of Citibank. At the time of the financial crash, bankers and financiers were seen as being in a state of moral hazard. Their job was outsourcing risk knowing that if things crashed, the bank would repossess your home. You would lose but they would still get bonuses. There was no incentive for them to be honest with you.
Doctors now also operate in a state of moral hazard. They put pills in your mouth and if these kill or maim you, you lose but they collect their pay. Things can only go wrong for them if they agree the treatment injured you. There is no incentive for them to do the right thing.
Slide 52: The result now is that we have a Pharmaceuticalization Crisis to rival the 2007 financial crisis. We have a polypharmacy pandemic. In 2007 money chased money as poverty rose, now drugs chase drugs as life expectancy falls.
Slide 53: We are in a state of hypnosis. Modern day sacraments are held up in front of us and we are told this is the way to Heaven. There doesn’t seem to be too much harm going along with this and so we slip innocently into a trance.
How do you break a trance? Exercising your judgement is key. You have to stick by your judgement it’s the drug that has made you suicidal or asexual. If you can be persuaded that your idea you are suicidal is caused by the drug is wrong and that this shows you are bipolar, and we need to add a mood stabilizer, then the trance deepens for you and your doctor. .
Slide 54: Science is on our side. The usual story about where science began points to 1660, when Charles II founded The Royal Society. The ground rule for science was it dealt with questions that could be settled with data.
Be you Xtian, Hindu, Jew, Muslim, or Atheist, a group of 12 or so had to describe what was happening in the event you saw in front of you and come up with a best possible explanation without appealing to an Authority. You could rerun the experiment, adapt the apparatus – but you couldn’t refuse to come to a consensus about the best explanation on a balance of probabilities basis about what you were seeing. The verdict might not be right and you were encouraged to experiment to check out both the observation and the explanation but with a view to coming to a new consensus.
Slide 55: This history overlooks an important earlier event, when Walter Raleigh had his head chopped off – for liaison with some Europeans. Raleigh was convicted on the basis of things said about him by people who did not come into court to be cross-examined.
The British legal system recognized an injustice and introduced Rules of Evidence. Hearsay was banned. The only evidence that counts has to be put in front of jurors who can see witnesses and material being examined and cross-examined. Twelve jurors are obliged to come to a consensus about what is in front of them.
This is the essence of science. Let’s say I give Pepe, who introduced this talk, an SSRI and he became suicidal on it. I can examine and cross-examine him, run lab tests and scans, raise the dose, stop the drug, add an antidote, check with colleagues if anyone has seen anything like this or can explain it without blaming the drug. Pepe may help if he has been depressed and suicidal before and can tell me this is different to what depression causes – and of course crucially if I put weight on his views, which I should – I shouldn’t have to add that in but its necessary in these times.
If Pepe and I conclude the drug has caused the problem and report this to FDA – you know what’s going to happen – the regulator will remove his name. No-one can now examine or cross-examine him or me and come to a scientific view about cause and effect. His Evidence has been transformed into Hearsay. When it comes to information medicines, FDA are now the greatest creators of misinformation on earth.
If you have a child or some injured on meds and say look there are 1000s of reports of suicides on SSRIs on FDAs MedWatch system, you will be told this is Hearsay and you cannot bring it into court.
The assays companies do to get their drug on the market are all Hearsay. No-one can be brought into Court to give evidence – the patients don’t always exist and the apparent authors have seen none of them . Names count. You are the data – the figures are just figures not data. Accessing the data means accessing you.
If Pepe and I report his case in a good journal like Ethical Human Psychology and Psychiatry, with our names on it, we can be brought into Court and examined in front of jurors. This is both clinical science and good evidence.
Slide 56: Today’s pressing medical need is to reduce our medication burdens. People are on 5, 10, 15 drugs and as medication burdens rise life expectancy falls. This still from the Hurt Locker movie maps onto the problem. Many of the bombs people are on explode on withdrawal. The psychotropic drugs post the biggest problems.
These meds are an experiment taking place in a person. S/he holds vital clues to the best way forward, and making progress involves coming to a consensus on this. RCTs and algorithms get us on drugs but can never get us off. The medicine we need now has to be relationship based rather than evidence based.
Slide 57: This image from the James Webb Telescope tells us that from genetics, to sub-atomic particles to astronomy real science proceeds toward individuality – we move from just being able to see the glow of the Milky Way and other galaxies to being able to pick out individual stars and this allows us to run experiments to find out more. The use of algorithms and RCTs in medicine is doing just the opposite – it collapses individuality.

Slide 58: For decades, people with PSSD wanted to remain anonymous. This played into company hands. Now groups like PSSD Network have gone public – challenging the media and the establishment to recognize them and tell us what they are doing to solve this terrible problem.
What we now call pharmacovigilance began with doctors like Louis Lasagna in the 1950s and was run by doctors through 1990 when pharma took over – then pharma passed the buck to regulators knowing they would do nothing. We can no longer depend on doctors or regulators to save us. We have to save ourselves. FDA and EMA aren’t going to do it for us – doctors aren’t going to do it – it needs you to do it.
To paraphrase a phrase:
It takes a village to raise a child – It takes a community to keep us safe on drugs.
Slide 59 You may not spot the beautiful woman here. King Arthur faced a duel with a Black Knight who wins and is about to kill him and then decides to spare his life if he can answer a riddle – What do Women Most Desire. He has a year to find the answer. The entire court hunt and fail and he is riding back to meet his death when they meet this woman by the edge of the road who tells him that she can give him the answer to the riddle but for him to have it one of his knights must become her husband. Gawain jumps down and offers himself up.
Slide 60 Arthur meets the Black Knight, gives him the answer and goes free. The next day Gawain gets married. Everyone at the Court is desperately unhappy for him.
Slide 61 In the bedchamber Gawain can’t look at her. She taps him on the shoulder and he finds a attractive woman. She asks – do you want me to look like this in the bedchamber or during the day in court or the other way around. With no idea what to say, he says – whatever you want. Which turns out to be the right answer.
She like us wants to control her own life. She may wants our support to treat a disease – but she doesn’t want me to tell her how to live a life, or want her negative emotions eliminated with a pill. There is a good chance she is doing better at living life than me.
Here is a strange and hard to put in words element of science – you have to believe in people, especially in this area of drug induced injury. Science depends on collaboration. The more you collaborate the more interesting and the more you like the people you collaborate with, and the more they like you.
Their motivation is worth as much or more than any expertise. For doctors its a choice between having lots of heartsink patients or lots of free research assistants – this should not be a difficult choice but seemingly is – there are structural barriers in the way.
Slide 62: Mikey Argy
I still get shocked when I see pictures of myself. I had a moment the other day when I was dosing in the sun on the beach, and I turned around and saw my hand and thought oooh that’s never going to change now. I think I believed that one day when I grow up my arms will become normal.
You can see Mikey’s right arm is not right – neither is her left. Still when last I saw Mikey – with Guy and Nick, all 3 born to mothers who had taken Thalidomide – there was 5 of 6 cups of tea to be picked up for a bunch of us and Mikey fetched them. No tray – I couldn’t have done it.
She was also just about to take her teenage daughters on a road trip – they didn’t drive then, she did. It was a cross-country trip – in a country where they drive on the wrong side of the road – they were about to cross the United States.
When you meet Mikey, Guy or Nick – you realise that those affected by Thalidomide have an amazing skill. They learn early on to hold your eyes so you engage with them and realise arms and things like that are irrelevant. These guys redefined what being able and being attractive and being human means.
Simon Roy and Bryn whom you met earlier are travelling the same route – learnings skills they never imagined they’d need, or putting skills they have to uses they never expected.
Psychotropic Drugs like the SSRIs haven’t just Simon, Roy, and Bryn but disabled our expectations of their expertise with unwarranted diagnoses like borderline personality disorder, ASD or bipolar disorder – conditions that none of these guys had. We are varied – some of us are risk-takers, extraverts, some are risk-managers, introverts, we need these differences just like a football team needs those who can score and those who can defend. But disabling us is part of getting us on these drugs.
Extraordinarily current research suggests that thalidomide and SSRIs have common effects on a key regulatory protein – p63. And it looks like research on proteins like this is a way to better antiviral and anticancer drugs. And doing research this way is more likely to give us drugs that will help us live the lives we want to live and not drugs designed to get us to live the lives companies want us to live.
It’s not me you should be listening to but Mikey, Guy and Nick, along with Simon, Roy and Bryn. You need to stop listening to claims linked to RCTs which in the case of Thalidomide tell you it works well and is entirely free of hazards.
Stop listening to the President of AMA or APA or the Royal College of Psychiatrists who recently told Simon when he got in touch to let her know he was enquiring about Euthanasia because of his PSSD. She told him that the sexual effects of SSRIs are transient and mild – nothing that a consult with a psychiatrist couldn’t put right for him.
Don’t adjust Your Personal Reality – it’s the virtual world that has gone mad.











































Compelling, powerful and immensely important. Thank you.
There is also the moral question of whether it would be a good thing to know what other people are thinking.
‘If not us. who are doctors listening to?
‘Simon outlines the impact of his PSSD on his family in an extraordinary fashion. What he outlines will be gripping listening for anyone interested in issues like this.’
The long, long journey of thoughts and where it can lead you, do you keep an iota of hope or is the position so overwhelming it can crush you?
The plight is almost unbearable.
This lecture takes us through Evident/Evidence based medicine and how we have reached the positions we are in. Introducing us to Roy, Bryn and Simon takes it to another level.
How much can the human mind take?
You lose something sacred to you, then to top it all you are dismissed as irrelevant.
‘A jury of 12 Wyoming folk with no background in health thought Hudson was Mad. They dismissed EBM in favor of Evident Based Medicine. It was obvious Paxil caused these homicides, they said, and GSK were guilty of negligence.’
‘Hudson, however, later became the CEO of Britain’s drugs regulator, and these Mad views are dug in at the top of FDA, EMA, and WHO.’
Don’t adjust Your Personal Reality – it’s the virtual world that has gone mad.
Random or Mad…
Ian Robert Burton Hudson OBE
The erstwhile Worldwide Director of Safety for SmithKline Beecham is now working full time for the Bill and Melinda Gates Foundation as fulltime Senior Advisor, Regulatory Affairs, Integrated Development.
In 2017, during his spell as CEO of the MHRA he negotiated a BMGF grant of $1.3m for “strengthening pharmacovigilance systems”.
Just before he entered the revolving door to BMGF they gave MHRA another grant – a mere $318,670 – to look at more effective use of medicines during pregnancy.
“Effective” from what point of view I wonder?
But it’s good to see they are concerned about pregnant people, and his well-documented background in drug safety will come in handy.
https://medregs.blog.gov.uk/2020/06/16/mhra-and-the-bill-and-melinda-gates-foundation-to-look-at-more-effective-use-of-medicines-during-pregnancy/
Always better in the flesh, the original
https://www.youtube.com/watch?v=cG4pk2Ib90M
74 views • Premiered 21 hours ago
This is a lecture given to the Annual Meeting of the International Society for Ethical Psychology and Psychiatry in October 2023
‘Doctors consume the drugs, by putting them in your mouth’ …
The Pitch…
3:15 PM · Oct 31, 2023
·
1,244 Views
Albert Bourla
@AlbertBourla
Today, as we share @Pfizer ’s third-quarter results, we also celebrate our far-reaching and positive impact on human health. In the first nine months of 2023, more than 457 million patients around the world were treated with our medicines and vaccines. And compared with the first nine months of 2022, we have reached more patients this year in several key therapeutic areas—including oncology, cardiovascular disease and anti-infectives. Patients will always be our North Star, and I’m proud to work with colleagues who are so committed to understanding and serving patients’ needs. While we saw an expected decline in revenues from our COVID-19 products during the quarter, we were encouraged by the continued strong performance of Pfizer’s non-COVID products, with revenue from these products growing 10% operationally compared with the year-ago quarter. We also continue to progress toward our goal of executing an unprecedented number of launches of new products or indications. To date, we have executed 13 of the 19 originally identified potential launches, with four more products approved and being prepared for launch. Among these launches are some historic firsts, such as the first and only RSV vaccine approved for the prevention of RSV in older adults and in infants via maternal immunization.
https://on.pfizer.com/3QFJeXI #PfizerProud $PFE
*Patient counts are estimates derived from multiple data sources.
Albert Bourla@AlbertBourla ·
Oct 30
RSV doesn’t discriminate on the basis of age. According to the CDC, each year, an estimated 60K-160K older adults in the US are hospitalized, and 6K-10K die due to RSV infection. The CDC also recognizes that RSV is the leading cause of hospitalization in infants. Talk to your healthcare provider about whether #RSV vaccination is recommended.
With so-called antidepressants being of the antihistamine class of drugs is it possible that widespread antihistamine use in general can lead to declining birth rates? I’m not well educated in drug mechanisms but in looking at slides 40-46 I wonder if the scope should be broadened past just antidepressants to other antihistamines as well. Any thoughts?
Its linked to serotonin reuptake. Roughly half of antihistamines inhibit serotonin reuptake but do so less potently than SSRIs so are less likely to cause problems. But there are also antidepressants like doxycycline and analgesics like tramadol than can inhibit serotonin reuptake and potentially contribute to problems
D
Yet again David Healy tells us like it is. His way of presenting the facts and his arguments are powerful and illuminating. Amazing work. Thank you.
Three of the top guns analyse new SSRI for ages 7 up
(Healy et al., 2018).
Eric Kuelker
@DrEricKuelker
·
1h
Good work. So depressing to see that a pill that is far better at inducing suicidal ideation than improving depression was approved by FDA. They really are the slaves, the puppets, the lapdogs of drug co’s,.
Pfluft @ploederl.bsky.social
@PloederlM
Our letter can be accessed for free the next 30 days: https://bit.ly/3PrF7g5
Pfluft @ploederl.bsky.social
@PloederlM
1/ Escitalopram now approved for generalized anxiety disorders for children & adolescents. FDA considers it as safe and effective for this new indication, based on a recent RCT. Let’s have a look at this trial -> twitter.com/CarlatPsych/st…
6:44 AM · Nov 3, 2023
1,652 Views
Martin Plöderl, Mark A. Horowitz , and Michael P. Hengartner
Journal of Child and Adolescent Psychopharmacology
To the Editor:
Recently, Strawn et al. (2023) reported on a clinical trial about escitalopram for the treatment of generalized anxiety disorder among children and adolescents. They concluded that “Escitalopram reduced anxiety symptoms and was well tolerated.” This conclusion is not supported by the data presented.
Efficacy, according to the primary outcome, is small, with a standardized mean difference of 0.27 (not reported in the article). None of the six secondary outcomes is statistically significant and the drug–placebo differences are small and even close to zero for the three measures for remission. This raises doubt about clinical significance of the therapeutic effects.
A major concern is suicide risk. In the acute treatment phase, 13 of 136 patients in the escitalopram arm reported suicidal ideation, compared with 2 of 137 in the placebo arm. The authors provided no statistical test, but the odds ratio (OR) is statistically significant, OR = 6.67, 95% confidence interval (CI) = 1.77–47.20, p < 0.01. Strikingly, the findings for suicidal ideation were not discussed at all.
There were also more adverse events other than suicidal ideation with escitalopram than with placebo (55.5% vs. 37.5%), which is statistically significant (OR = 2.07, 95% CI = 1.28–3.37, p < 0.01, not reported in the article). Furthermore, sexual dysfunctions were not systematically assessed but it is known that these are rarely spontaneously reported and occur at much higher rates with antidepressant than with placebo (Serretti and Chiesa, 2009).
Finally, it is well known that adverse events are under-reported in clinical trials (Golder et al., 2016) and methodological biases may lead to an overestimation of efficacy in antidepressant trials (Healy et al., 2018).
Consequently, in this trial, the efficacy of escitalopram is likely not clinically meaningful, and the increase of suicidal ideation and other adverse effects suggests that the drug conveys significant harms. Relative to treatment with placebo, 8% more patients treated with escitalopram developed suicidal ideation (1.5% placebo vs. 9.5% escitalopram) but only 6.2% more patients achieved clinical response (33.3% vs. 39.5%) and 1.5% fewer achieved remission (17.7% vs. 18.8%). Thus, relative to placebo, children and adolescents exposed to escitalopram were more likely to become suicidal than to experience an improvement in anxiety. These results are concerning and the conclusions in the article seem to be misleading.
My life was ruined by being left on these experimental drugs for over three decades! Experimental science and profit over the people. Complete Madness!
Thanks for this great article. ”Extraordinarily current research suggests that thalidomide and SSRIs have common effects on a key regulatory protein – p63.” Do you think this might be relevant regarding PSSD? Where can I find about this more?
There is lots about this on RxISK.org.
Look under the sex category or on the PSSD tab on resources
D