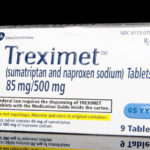
The plan this morning was to continue coverage of the Opioid Epidemic but perhaps because it is Halloween a link to a Wall Street Journal story on how Drug Makers Turn Cheap Generics into Expensive Pills arrived by email. It was difficult to resist. Here's why. The article features Treximet, a combination of sumatriptan and naproxen, used for migraine. These two drugs are … [Read more...] about Study 329 Trick, Treat or Treximet
Study 329
Go Figure: Study 329
Editorial Note: This post merges the Go Figure sequence of posts from several weeks ago with the 329 series. In the wake of the French Revolution of 1968, the government was still tottering when on February 4 1970, sixteen miners died with twelve others maimed in an explosion at a mine in Lens in France. The mine owners pitched the event as an accident. It didn’t help their … [Read more...] about Go Figure: Study 329
Study 329 Taper Phase
Editorial Note: It was tempting not to run a post today for fear it might get lost in the wash of the Clinton-Trump debate. But today is the fourteenth anniversary of the day FDA issued an approvable letter for Paxil for children, as well as the fifty-fourth anniversary of the 1962 FDA Act that created the playing field on which Study 329 happened. It's also World Mental Health … [Read more...] about Study 329 Taper Phase
Study 329 Continuation Phase
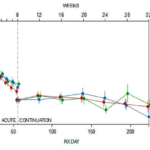
Editorial: We interrupt the Go Figure series of posts to return for two posts to Study 329. We will then return to Go Figure. All the fuss about Study 329 centers on its 8 week acute phase. But this study had a 24 week Continuation Phase that has never been published. Until Now. We might have Marty Keller to thank for this Continuation Phase. His big deal was the long term … [Read more...] about Study 329 Continuation Phase
Go Figure: Sally’s Problem with Whinging about Medicines
Two weeks ago in response to the last post in the Study 329 series, Sally MacGregor added the comment - that features as a post below. It's spot on. The problem is how to avoid being marginalized, becoming part of a 1%. How to capture the attention of the 99% for whom the meds work just fine thanks. There will be more on this theme over the next few posts. The whole point … [Read more...] about Go Figure: Sally’s Problem with Whinging about Medicines
Club 329: Part 4
Editorial Note: This post perhaps should be called: There's Something about Leonie. The image above is of a Rapid Response she submitted to a BMJ editorial by Richard Smith and Fiona Godlee that BMJ published and unpublished and republished and re-unpublished. The full story is here. It again hinges around Study 329. The full transcript of her exchanges with Ben G is below. … [Read more...] about Club 329: Part 4
Club 329: Part 3
Editorial Note: This post by Leonie Fennel carries on from parts 1 and 2 in this series. There will be one more post. I dreamt I met my son Shane last night - in a jewelry shop, of all places. I was admiring the beautiful costume jewelry, when I overturned the dainty display and went clambering to pick up all the pieces. It seems I don’t escape my klutziness in the land of my … [Read more...] about Club 329: Part 3
Club 329: Part 2
Following last week's post, Club 329: Part 1, Ben Goldacre went into orbit claiming his views on medicalization and Study 329 had been misrepresented. He offered a SoundCloud as evidence. The link can be found in the comments after the last post. Seems to me Leonie got the content of the Q & A right. In the course of listening to the BG SoundCloud though something else … [Read more...] about Club 329: Part 2
Club 329: Part 1
Editorial Note: This post is by Leonie Fennel. It's one of two involving Leonie. Last week, Dr Ben Goldacre gave a public lecture in the Royal College of Surgeons in Dublin (organised by the 3U Partnership and the very lovely Dr Ruth Davis). Dr Goldacre is a doctor, academic, campaigner and writer; he is also a psychiatrist and self-professed nerd. I was eager to hear what he … [Read more...] about Club 329: Part 1
Restoring Study 329: Letter to BMJ Jan 2016
Editorial Note: This was our final letter in the correspondence that began here and ran through here. There has been no response from Dr Godlee. 28 January 2016 Dr Fiona Godlee Editor, BMJ Re: BMJ handling of conflict of interest Dear Dr Godlee Thank you for your email response of January 11 2016 to our letter of July 8 2015 about Dr Elizabeth Loder's conflict of … [Read more...] about Restoring Study 329: Letter to BMJ Jan 2016