
Big Friendly Gates
On 28 April the NEJM published the interim results of a trial of Prefusion F Protein–Based Respiratory Syncytial Virus Immunization in Pregnancy. This paper contains evidence of a neonatal jaundice hazard in the babies whose mothers had been immunised – but you’d never guess it from the way the paper is written.
Prizer’s RSVpreF Trial
Pfizer’s phase 2 trial NCT04032093, run among other placed in three Ventavia sites in Texas, enrolled 1153 pregnant women. They were either injected with RSVpreF or placebo. Antibody levels and adverse events were recorded in them and their babies.
What is Prefusion F Protein – PreF? Who knows. The published paper says this was an immunization trial, aimed at producing antibodies. That’s not the same as traditional vaccination or treatment with a monoclonal antibody or an mRNA type agent.
The NEJM report names Eric Simões as first author but it is unlikely to have been written by him. It concluded:
“RSVpreF vaccine elicited neutralizing antibody responses with efficient transplacental transfer and without evident safety concerns.”
Yet the supplement to Simões’s article shows more than twice as many babies developed jaundice in the vaccinated group compared with the placebo group (59/325 v 6/78). In nine of the vaccinated babies this was deemed a serious adverse event, compared to one in the placebo group.
Jaundice in new-born babies is fairly common but isn’t very nice and can be serious. Too much bile pigment (bilirubin) in the blood stains the babies’ skin and the whites of their eyes deep yellow. It’s bad news if it gets into the brain (kernicterus).
The Table below shows a statistically significant increase in Jaundice in babies of vaccinated mothers compared with placebo group. It is derived from Table S8 of the supplementary appendix to the Simoes article.
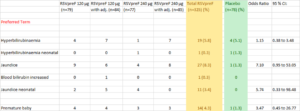
By diluting adverse events among four different vaccine formulations this chart semi-hides, a total of 11 instances of “Jaundice neonatal” in the babies of vaccinated mothers, compared with none in the placebo group.
Further creativity is shown by distributing the diagnosis of jaundice under a range of other terms – “Hyperbilirubinaemia”, “Hyperbilirubinaemia neonatal”, “Jaundice” and “Blood bilirubin increased”. Taking all these into account, the totals become 59/325 on Pre F versus 6/78 on Placebo – more than double the rate.
Premature birth is also a lot more common in the vaccinated group.
According to the protocol the babies in this study had three “blood draws” during the first 12 months of life – to determine antibody levels: liver function was not checked.
We have no idea what Eric Simões, Pfizer’s Internal Review Committee and the External Data Monitoring Committee for this trial make of this apparent warning signal.
It is almost for certain others have noticed this hazard and Dr Eric Rubin, editor of NEJM which published Simões’s report, must have been contacted but there has been no published correspondence to date – over three months later.
Eric Rubin Billy Rubin
We have not been able to find any reports of RSVpreF being tested in pregnant animals so as to monitor any effects on the offspring.
We wrote to alejandra.gurtman@pfizer.com about the animal testing and several months later have had no response.
RSV and Bronchiolitis
Before Covid was, RSV was.
Respiratory Syncytial Virus (RSV) is one of the commonest causes of Bronchiolitis, a lung disease of infants affecting about 3% of children in their first year of life, usually in the winter. The RSV virus was isolated in 1956 from chimpanzees and then from children. By the age of two, most children have been infected with it.
Most of these infections are mild. Children recover at home without specific treatment. There is no evidence that antibiotics, steroids, bronchodilator drugs and routine oxygen therapy are of benefit. Chest X-rays are unnecessary. The most poorly children will need to be admitted to hospital for supportive care.
Since 1990, according to WHO, the all cause of death global under-5 mortality rate has dropped by 60%, from 93 deaths per 1,000 live births in 1990 to 37 in 2020, despite Covid.
This explains how studies can now claim RSV infection as the cause of an increasing proportion of childhood deaths. RSV deaths are not increasing but as other causes of death fall it can be spun as an increasing cause for concerns.
RSV is also implicated as causing pneumonia in the elderly. Several vaccines are being trialled. In contrast to Covid, where children were vaccinated to save their grandparents, this time the propaganda will be directed at grandparents – or perhaps at mother telling her not to let grandparents visit unless they have been vaccinated.
See Reds under the Bed and this Oh Granny video
Raiders of the Lost Vaccine
Since the 1960s hundreds of millions of dollars have been pumped into finding a vaccine to prevent RSV infection.
A 1966 field evaluation of a formalin-inactivated RSV vaccine was conducted by the NIH in a selected US paediatric population from relatively low socioeconomic status, primarily Afro-American families, using Pfizer’s experimental RSV vaccine designated “Lot 100”. Despite good antibody responses to the immunisations there was no effect on the incidence of bronchiolitis.
Over the following two years, those children who received the vaccine were sixteen times more likely to suffer in hospitals compared to controls. Pfizer’s 1965-6 RSV vaccine had: “Disastrous results”. Two of the 31 vaccinated toddlers died from a severe form of bronchopneumonia known as Vaccine-Associated Enhanced Respiratory Disease (VAERD). It would be nice to think that the families were given compensation.
In 2016 Dr Fernando Polack, of So Long and Thanks for all the Fish fame, co-authored an article on VAERD, sponsored by BFG, stating that these toddlers died as a consequence of subsequent RSV infection, rather than due to an adverse event triggered by the vaccine. Pfizer is not mentioned.
Vaccine development stalled after the VAERD tragedy. But the “race” to make money out of RSV is back on with a vengeance. There are currently nineteen RSV trials in children and adults listed as in progress.
The Harrison Fords of RSV research are.

Prof. Harish Nair Prof. Eric A F Simões Dr Fernando Polack
Harish Nair is Chair of Paediatric Infectious Diseases and Global Health and leads the Respiratory Viral Epidemiology research programme at the University of Edinburgh. He is the coordinator of RESCEU – see below).
Eric Simões is Clinical Professor, Pediatrics-Infectious Diseases in the University of Colorado School of Medicine. He received more than $4M from Pfizer during 2021 for research and was lead author of Pfizer’s recent antenatal RSVpreF vaccine trial.
Bi-lingual Fernando Polack runs his Florida/Argentina/Uruguay clinical trials company I-TRIALS SA. The BFG invested $82,553,834 into Novavax’s RSV vaccine ResVax. Fernando ran the trial. ResVax turned out to be ineffective when administered to pregnant women, failing to prevent medically significant RSV in their babies.
(A rear-guard action claims that the vaccine reduced the “burden” of antimicrobial resistance as apparently the babies of immunised mothers were prescribed fewer antibiotics in the first three months of life.)
Just as the Novovax results were being announced, Covid came along and Polack side-stepped into the Prizer Covid vaccine trial. His company hired 467 local doctors as investigators and recruited nearly 6000 volunteers in Buenos Aires for Pfizer’s Comirnaty covid trial.
I-Trials has since been looking at combination Covid. RSV and Influenza vaccines – to be given annually.
In February 2021, GSK quietly dropped out of the RSV race after their trial vaccine for babies ChAd155-RSV was found ineffective. In February 2022, GSK’s trials in pregnant women with “RSV MAT” vaccine were stopped when it flagged up safety signals. We still don’t know what these are.
While there are nineteen vaccine trials in progress, at moment the race to scoop the RSV jackpot is between AstraZeneca/ Sanofi’s nirsemevab, the bookmakers’ favourite, and Pfizer’s RSVpreF – neither of which are vaccines.
Nirsemevab
Two recent NEJM papers describe the outcomes of “pivotal” (i.e. for FDA) trials of Nirsemevab, an injectable monoclonal antibody against RSV.
Is Nirsemevab a vaccine? No. See AstraZeneca explanation.
The primary endpoint in the trial is “medically attended RSV-associated lower respiratory tract infection”. This is strange as, as stated above, the majority of medical interventions take place outside of hospitals and have few, if any, benefits.
There were 3 deaths, all in the nirsemevab group.
Here are a couple of tables in the supplementary appendix of one of the trial reports.
It seems that if children who had been given Nirsemevab were admitted to hospital with RSV, their hospital stays were 50% longer than in the placebo group.
Table s6
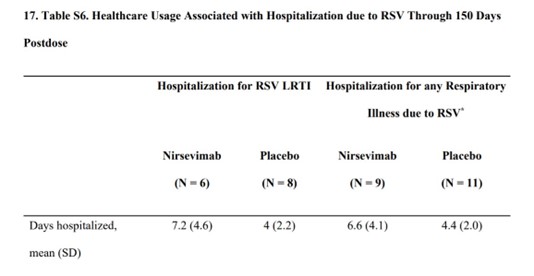
Table S8 is an example of two recorded Grade 3 adverse events – but were they in the treatment group or the control group? Astra-Zeneca won’t say.
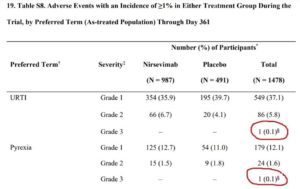
It looks likely that Nirsemevab is linked to the more serious events but the company say
“Tabulations that present single or multiple participants with AEs in only one study arm (nirsevimab or placebo) are presented across both study groups to preserve the study blind.”
The trial actually is over and one of us reached out to tonya.villafana@astrazeneca.com and asked whether or when the results would be updated. No response.
According to several recent papers in the Lancet – see below – there were 33.0 million RSV-associated acute lower respiratory infection episodes and 3.6 million RSV-associated hospital admissions, globally in 2019.
If the condition is so common, why did the nirsevimab trial need 215 centres in 34 countries including, for example, USA, Ukraine, Argentina and Panama (wrongly located in the Southern Hemisphere)?
This trial offers another great example of how companies hide hazards. Well – This comes to pass when a child is coughing. Just like any hint of jaundice above, the cough can be coded under or later put into any of the following “preferred term” diagnostic categories?
- Atypical pneumonia,
- Bronchiolitis,
- Bronchitis,
- Bronchitis viral,
- Croup infectious,
- Laryngitis,
- Lower respiratory tract infection,
- Lower respiratory tract infection bacterial,
- Lower respiratory tract infection viral,
- Nasopharyngitis,
- Pharyngitis,
- Pharyngotonsillitis,
- Pneumonia,
- Pneumonia bacterial,
- Pneumonia parainfluenza viral,
- Pneumonia respiratory syncytial viral,
- Pneumonia viral,
- Respiratory syncytial virus bronchiolitis,
- Respiratory syncytial virus bronchitis,
- Rhinitis,
- Subglottic laryngitis,
- Tonsillitis,
- Tracheobronchitis,
- Upper respiratory tract infection,
- Upper respiratory tract infection bacterial,
- Viral pharyngitis,
- Viral rhinitis,
- Viral upper respiratory tract infection,
- Bronchial hyperreactivity,
- Bronchitis chronic,
- Bronchospasm,
- Catarrh,
- Pneumonia aspiration,
- Respiratory failure,
- Respiratory symptom,
- Wheezing.
or
- Cough
Let some coders loose on the job and most hazards in clinical trials disappear. You don’t even need to tell the coders you want things to disappear.
At a recent US Advisory Committee on Immunisation Practices, trying to get nirsemevab approved, Sanofi’s Dr Christian Felter introduced a slide to show the difference between 2% and 100%. Has he overestimated the intellect of the Committee on Immunisation Practices?

Afterwards Dr Katherine Poehling asks some searching questions about deaths and grade 4 adverse events. Dr Amanda Leach O.B.E. for AstraZeneca does her best to fend these off. The ticker taper says there were no fewer deaths in the vaccinated group but actually Listening to Amanda is likely to leave some of you pretty sure that nirsemevab is far from safe.
(Dr Felter’s presentation is at 55:15 in the Listerning to A Video.)
If approved Nirsemevab will not be the first monoclonal antibody approved for RSV. Palivizumab has been approved for a decade. It has a good safety record and reduces bronchiolitis.
Humanized monoclonal antibodies (palivizumab) have been used for many years for the prevention of respiratory syncytial virus infection in pediatric populations (preterm infants, infants with chronic lung disease or congenital heart disease) at high risk of severe and potentially lethal course of the infection.
Giving Pavilizumab in this way might be a sensible use for a monoclonal antibody. Does AstraZeneca envisage restricting the use of Nirsemevab in this way?
Shi T Happens
It is hard to find data about the impact of RSV that is not tainted by drug company / BFG money. See for example Shi T., Polack F et al, funded by BFG, another paper telling us that there are 33 million cases of RSV in children worldwide, leading to over 3 million hospitalizations and nearly 60,000 deaths. This was also published in the Lancet. The figures are ‘estimated’.
There are two main funding streams for RSV “research”
- The Innovative Medicines Initiative (IMI), recently rebranded as The Innovative Health Initiative (IHI). Half of its funding comes from European Federation of Pharmaceutical Industries and Associations (EFPIA) – amounting to €1.638 billion for the period 2014-2024. In addition, EFPIA is committed to contribute €1.425 billion of “in-kind contributions”.
IMI funds PROMISE – Preparing for RSV immunisation and surveillance in Europe – to the tune of €7.024 billion as well as RESCEU – REspiratory Syncytial virus Consortium in EUrope “The RESCEU project aims to develop robust evidence on RSV disease burden and economic impact in Europe and provide infrastructure to perform future pivotal clinical trials for RSV vaccines and therapeutics” .
IMI also funds conect4children “Better medicines for babies, children and young people through a pan-European clinical trial network.”
- The BMGF: the mellifluous Bill & Melinda Gates Foundation. This props up Fernando Polack’s Argentine Fundación Infant and also glossy RSV Gold – a Grim Reaper’s RSV Global Online Mortality Database which aims to collect as much bad news as possible about RSV in children.
- Gates and vaccine manufacturers additionally finance the Respiratory Syncytial Virus Foundation annual conferences, as does PATH “we are a global nonprofit improving public health” which in turn receives drug company loot.
Losing the Plot ?
Children may be more at risk from current RSV vaccine trials, and in due course RSV vaccines or monoclonal antibodies, than they are from RSV:
- Nirsemevab causes more deaths than placebo in controlled trials.
- In the Nirsemevab trials, serious adverse effects are being hidden.
- Neonatal jaundice and prematurity are more common in neonates born to mothers given RSVpreF.
- Were there any safety trials of RSVpreF in pregnant animals – if so, where are they?
- What problems did GSK’s antenatal RSV vaccine cause? These must be disclosed.
- Researchers and Journals have stopped engaging in any discussion of concerns.
The biggest worry of all might lie in the early history of RSV vaccines – which put the concept of VAERD Vaccine Associated Enhanced Respiratory Disease on our radar
This is now recognised with Dengue and other Vaccines. In the case of Covid and its vaccines we now have Neonatal Multi-System Inflamatory MIS-N. See Karunya Come Home for more on MIS, and MIS-C.
The severity of RSV peaks at the age when infants have high maternal neutralising antibodies.
Vaccine Preventable
Conditions like RSV are now labeled vaccine preventable. If something becomes VP, it seems all sense flies out the window. No-one stops to ask whether there may be more harm in indiscriminately preventing infection than in leaving things alone or selectively intervening as with Palivizumab.
Nobody stops to ask whether there could be problems giving new treatments about which we know very little in pregnancy. Since the mid 1970s there has been a huge increase in drugs taken in pregnancy – by wealthier, older, college educated and white women. This looks likely to repeat with vaccines.
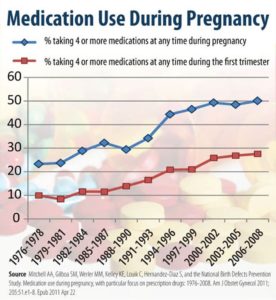
This gameplan maps on to the Risk Prevention approach in medicine with minimal, almost non-existent, risks like cholesterol levels targetted because we can but with no-one asking whether poisoning 100 healthy people with drugs that will injure or kill many of them is a good idea in order to save one life from a cholesterol linked risk or other risk linkage.
When we then end up with people on ten drugs to manage ten notional risks we brew up a cocktail that is shortening our lives, increasing hospitalizations, leading to health service collapse, and giving us a far worse quality of life. See Shipwreck of the Singular.
This way madness lies.
There will almost certainly be unexpected problems with these new vaccines – like Achilles tendon ruptures on fluoroquinolone antibiotics or suicide on doxycycline – which need recognition but will now be denied as having any links to these vaccines – see Harmatology.
This way lies a destruction of healthcare from New Zealand, through Australia and Europe to North America. Health services are on their knees with politicians telling us the answer is even more high tech. Common Sense just doesn’t appear common any more.
####
Two days after this post went up, the Lancet carried a new BFG funded paper on the horrors of RSV and the absolute necessity to have 33 trials currently running on vaccine and monoclonal antibody development in this area – for pregnant women, infants and older adults.


If infants are prevented from learning how to cough in order to expel viruses naturally ,apart from weakening their immune system will the vaccine not be preventing them from developing the muscles , the physiology needed for coughing and for developing t strong lungs necessarily for a healthy active life?
Another Congested Race…Give it to Mum…
“A big concern for the scientists involved in RSV vaccine development is to make sure we do not repeat the same situation again,” said Polack, who is also affiliated with the INFANT Foundation in Buenos Aires.
Research shows why 1960s RSV shot sickened children
https://www.reuters.com/article/us-rsv-shot-idUSTRE4BM4SH20081223
NEW YORK (Reuters Health) – Researchers from Johns Hopkins have solved the decades-old mystery of why a vaccine developed to prevent a common childhood viral infection wound up making kids sick.
The findings provide important clues to how to develop a safe, effective vaccine against respiratory syncytial virus (RSV), the main cause of wintertime hospital stays among babies and young children worldwide, Dr. Fernando P. Polack, the lead researcher on the study, told Reuters Health.
“A big concern for the scientists involved in RSV vaccine development is to make sure we do not repeat the same situation again,” said Polack, who is also affiliated with the INFANT Foundation in Buenos Aires.
Vaccine failure explained
Immunologists show how deaths in 1966 could have been avoided.
https://www.nature.com/articles/news.2008.1302
“MedImmune has made huge amounts of money on Synagis,” says Openshaw.
“There are some vaccines that came very easily, but developing a vaccine for RSV is very tricky,” says Polack. But, he adds, a vaccine for the disease is urgently needed.
https://www.fiercebiotech.com/biotech/gsk-drops-phase-2-viral-vector-rsv-vaccine-pediatric-ages
The RSV vaccine, GSK3389245A, also known as ChAd155-RSV, is a recombinant chimpanzee adenovirus vector vaccine that GSK developed with the Oxford Vaccine Group—also known for working on AstraZeneca’s COVID-19 jab. The vaccine is based on three RSV viral proteins encoded by the vector and was expected to engage both the humoral and cellular arms of the immune system against the pathogen.
“GSK remains committed to addressing the burden of RSV disease through our maternal and older adult vaccine candidates, which use different technologies … and are both progressing in phase 3 trials,” a spokesperson said. “We remain confident in the potential of our viral vector technology, and our therapeutic hepatitis B virus development program continues as planned.”
News of the discontinuation comes months after GSK began a late-stage study of another vaccine for RSV, GSK3888550A. By giving the vaccine to pregnant women, GSK is aiming to protect babies from the virus during their first months of life.
Pfizer raises pressure on GSK, J&J with late-stage RSV vaccine trial
https://pharmaphorum.com/news/pfizer-raises-pressure-on-gsk-jj-with-late-stage-rsv-vaccine-trial/
Moderna recently started trials of an mRNA-based flu vaccine candidate, and said it intends to move ahead with a plan to combine flu, COVID-19 and RSV into a single shot. Pfizer meanwhile has a partnership with its COVID-19 vaccine compatriot BioNTech that also covers an RSV jab.
While there is no approved RSV vaccine yet, drugs are available to treat patients at the other end of the age spectrum – infants and very young children – who are also at risk of serious complications from the virus.
Swedish Orphan Biovitrum (Sobi) currently sells Synagis (palivizumab), a monthly antibody which is the only approved drug to provide passive prophylaxis against the virus in paediatric patients, while another antibody candidate from AstraZeneca and Sanofi is headed for regulatory filings next year.
GSK meanwhile is also exploring whether its vaccine could impart protection to children if administered to their mothers before birth.
Edward Dowd Reposted
rwmalonemd
@rwmalonemd
Vaccines Are Bringing Back a Nearly Eradicated Deadly Virus
https://link.theepochtimes.com/mkt_app/vaccine…
Vaccines Are Bringing Back a Nearly Eradicated Deadly Virus
BY XIAOXU SEAN LIN
https://www.theepochtimes.com/mkt_app/vaccines-are-bringing-back-a-nearly-eradicated-deadly-virus_4650115.html
However, vaccines are not medications, and they don’t function to “kill” a virus. It only help to stop the transmission of the virus in ideal situations.
We don’t know of any other natural animal reservoirs for the poliovirus to hide, outside human hosts. How could we “eradicate” a certain virus with vaccines when we don’t know where the virus comes from?
Are we creating more problems with a wrong objective coupled with a wrong approach?
On the one hand, people want to wipe polio out from the face of the earth, yet on the other hand, the vaccines are “spreading” the viruses all over the world. The VDPVs are still polio viruses, even though they are not wild type polioviruses. Delta or Omicron variants are still SARS-CoV-2 viruses, even though they are not the wild type Wuhan strain. Are we fooling ourselves by declaring some regions or countries as polio-free, simply based on not detecting wild type poliovirus, while we clearly know that VDPVs are still circulating, and even spreading faster due to the large OPV campaigns in many developing countries?
With billions of dollars spent on GEPI globally, with many intensive campaigns to inoculate people, especially children, with OPVs (regardless old or novel versions), we are inoculating the world with more VDPVs in nature as we have no way to contain its existence and spread. Is eradicating polio becoming a delusion that the world just cannot wake up from, as we have invested so much of money, emotion, effort, and dedication to it, even though we know that the train is on the wrong track?
Children under 10 will get polio boosters as virus returns to UK for first time in 40 years
https://www.dailymail.co.uk/news/article-11093247/Children-10-polio-boosters-virus-returns-UK-time-40-years.html
There have been no confirmed cases of polio in patients in Britain so far – but experts have suggested it has been passing from person to person because of the amount of poliovirus samples in sewage.
The virus was detected at the Beckton sewage treatment works, which covers a population of four million in north and east London.
It is normal for sampling to detect one-off traces of poliovirus in sewage each year, but officials said a sample identified in April was genetically linked to one first seen in February which persisted and mutated into a ‘vaccine-derived’ poliovirus, which is more like the ‘wild’ type that can cause serious symptoms.
Lyme Disease Vaccine: Pfizer Launches Phase 3 Trial Targeting Kids, Adults
https://childrenshealthdefense.org/defender/pfizer-valneva-lyme-disease-vaccine-clinical-trial/
If approved, the vaccine could be the first human vaccine available for Lyme disease in the U.S. in more than two decades after LYMERix, manufactured by GlaxoSmithKline, was withdrawn from the market in 2002, due to lawsuits, safety concerns and dwindling sales.
Similar to claims Pfizer made about booster doses of the COVID-19 vaccines, the drugmaker said that while the two-dose regimen of VLA15 demonstrated immunity, a third VLA15 dose “increased the level of antibodies against an outer surface protein.”
According to a press release, Pfizer and French partner Valneva are enlisting 6,000 participants ages 5 and older for a late-stage clinical trial that will test the vaccine, VLA15, against the tick-borne illness.
According to ClinicalTrials.gov, “18,000 healthy participants 5 years and older” were recruited for the study. In its press release, Pfizer did not explain the discrepancy in the number of trial participants.
How the LYMErix Lyme Disease Vaccine was Pulled from the Market
https://www.lymedisease.org/members/lyme-times/special-issues/tick-borne-disease/lymerix-lyme-disease-vaccine/
“It’s rare that a vaccine be voted on with such ambivalence and a stack of provisos.”……
There is a real gap in the market for children aged between age 3½ (MMR (2nd dose) +
4-in-1 pre-school booster*) and age 11 (HPV vaccine).
Those UK kids must feel left out.
* wow: SEVEN antigens in one go! So physiological.
The primary factor behind these vaccine schedules appears to be convenience rather than science. There is nothing natural about giving a baby 6 infections along with adjuvants etc all in one go at 6 weeks of age and then repeating the trick and few weeks later.
A colleague was telling me her doctor musing with her rather than trying to persuade or convert said it was his impression that delaying these early vaccines by some weeks seemed to lead to less bronchiolitis subsequently.
We also don’t know for instance ssimple things like what makes more sense – sticking to a rigid 6 week after birth schedule when say the baby was born premature or aiming at vaccination 6 weeks after the due date? There is a big difference between the two but it seems most likely that no-one is in a position to offer a physiologically informed view on what might be best.
DH
‘Acting at speed ‘
we believe the public will understand and thank us for this decisive action’. Clap, clap
.
Navigation menu MenuSearch GOV.UK
Home
Letter to the profession from the UK Chief Medical Officers on the UK COVID-19 vaccination programmes
Department of Health & Social Care
31 December 2020
Dear colleagues,
Thank you for your remarkable commitment to the health of our nation in the most difficult of circumstances; the COVID-19 pandemic is undoubtedly the biggest health crisis in a generation, and certainly in our professional lifetimes. We are at a critical point in the pandemic as the emergence of a novel variant of SARS-CoV-2 with a markedly higher growth rate is rapidly shifting the epidemiological curve in the wrong direction across much of the UK in the middle of winter.
Authorisation of first the Pfizer and now the AZ vaccine (AZD1222) for use is incredibly welcome. Both are highly effective vaccines from clinical trial data and are anticipated to have sizeable effects on preventing severe disease and hospitalisation. Getting vaccines deployed as rapidly as possible into as many older, clinically vulnerable patients, and also frontline health and social care workers is essential. The Joint Committee on Vaccination and Immunisation (JCVI) has put forward a prioritisation scheme, attached, of which you will all be aware.
We wanted to lay out to you the scientific and public health rationale for the dosing schedule for the AZ vaccine and the change to the dosing schedule for the second dose of the Pfizer vaccine. As with all decisions during this pandemic it is about balance of risks and benefits.
We have to ensure that we maximise the number of eligible people who receive the vaccine. Currently the main barrier to this is vaccine availability, a global issue, and this will remain the case for several months and, importantly, through the critical winter period. The availability of the AZ vaccine reduces, but does not remove, this major problem. Vaccine shortage is a reality that cannot be wished away.
We are confident that based on publicly available data as well as data available to the JCVI, the statutory independent body, that the first dose of either Pfizer or AZ vaccine provides substantial protection within 2-3 weeks of vaccination for clinical disease, and in particular severe COVID disease. The JCVI has issued a new evidence statement today.
The second vaccine dose is likely to be very important for duration of protection, and at an appropriate dose interval may further increase vaccine efficacy. In the short term, the additional increase of vaccine efficacy from the second dose is likely to be modest; the great majority of the initial protection from clinical disease is after the first dose of vaccine.
In terms of protecting priority groups, a model where we can vaccinate twice the number of people in the next 2 to 3 months is obviously much more preferable in public health terms than one where we vaccinate half the number but with only slightly greater protection.
This is why the JCVI has recommended that first doses of vaccine are prioritised for as many people as possible on the Phase 1 JCVI priority list, in advance of second doses which will subsequently provide more assured longer-term protection. It is a classic public health approach centred on doing as much good for as many people in the shortest possible timeframe, within the available vaccine supplies, against a background of immediate disease activity and still high population sero-susceptibility (despite the disease burden seen).
The JCVI is confident 12 weeks is a reasonable dosing interval to achieve good longer-term protection.
The position is strongly supported by the UK Chief Medical Officers on public health grounds of maximising benefit.
We recognise that the request to re-schedule second appointments is operationally very difficult, especially at short notice, and will distress patients who were looking forward to being fully immunised. However, we are all conscious that for every 1000 people boosted with a second dose of COVID-19 vaccine in January (who will as a result gain marginally on protection from severe disease), 1000 new people can’t have substantial initial protection which is in most cases likely to raise them from 0% protected to at least 70% protected. Whilst the NHS, through all of your work, has so far vaccinated over 1 million UK patients with a first dose,approximately 30 million UK patients and health and social care workers eligible for vaccination in Phase 1 remain totally unprotected and many are distressed or anxious about the wait for their turn. These unvaccinated people are far more likely to end up severely ill, hospitalised on in some cases dying without vaccine. Halving the number vaccinated over the next 2-3 months because of giving two vaccines in quick succession rather than with a delay of 12 weeks does not provide optimal public health impact.
We have to follow public health principles and act at speed if we are to beat this pandemic which is running rampant in our communities and we believe the public will understand and thank us for this decisive action. We hope this has your support.
GOV.UK
Navigation menu MenuSearch GOV.UK
Brook Jackson
@IamBrookJackson
·
Aug 13
The Time of COVID –
Dr. Phillip Altman There is even more explosive information and documents that will be released in the coming days!
Please share
https://8630368.fs1.hubspotusercontent-na1.net/hubfs/8630368/AMPS/Altman%20Report%20Final%20Version%2011-8-22%20(1).pdf?utm_source=hs_email&utm_medium=email&_hsenc=p2ANqtz-9QLZBYAx-iWryax8LzJZbPBhwdHOK0WZCH4DH10l4iSkGPO-AROeYGrSVybiel3ygZuKtL
Given the statistically or virtually nil risk of serious COVID-19 in general affecting children aged 6 months to 11 or 12 years of age and the clear and significant risk of serious adverse effects including myocarditis, pericarditis and death in this age group – there seems to be little benefit to be gained by vaccinating these children.
Considerable scientific, clinical and statistical epidemiological data and understanding has been acquired since the introduction (on a provisional basis only) of the investigational COVID-19 gene-based “vaccines”. Many of the initial ambitious claims and assumed perceptions regarding the safety and efficacy of these serious therapeutics have now been invalidated and it is now time to review and reconsider the utility of these products in light of the known unprecedented level of serious adverse reactions and death attributed to their use.
—
If we’ve all been jabbed and millions have had the virus at least once – why are so many of us being poleaxed by a bout of Covid?
13 August 2022
https://www.dailymail.co.uk/health/article-11108521/Covid-19-weve-jabbed-poleaxed-virus.html
This all begs the question: why, more than two years after Covid first appeared, are so many of us suddenly being laid so low?
Experts agree that the decision to offer all adults a third jab last winter, in response to the arrival of the highly transmissible Omicron variant, was a success.
This is very much an important read, had trouble with this link so hope this works –
The Time of COVID
A Report by Phillip M. Altman
BPharm(Hons), MSc, PhD
Clinical Trial & Pharmaceutical Regulatory Affairs Consultant
9 August 2022
https://8630368.fs1.hubspotusercontent-na1.net/hubfs/8630368/AMPS/Altman%20Report%20Final%20Version%2011-8-22%20(1).pdf?utm_source=hs_email&utm_medium=email&_hsenc=p2ANqtz-9QLZBYAx-iWryax8LzJZbPBhwdHOK0WZCH4DH10l4iSkGPO-AROeYGrSVybiel3ygZuKtL
‘It is also hoping to have a Covid-flu combined vaccine ready by next winter, as well as a triple-threat jab combining Covid and flu with respiratory syncytial virus (RSV) by the winter of 2024-25.’
UK authorises ‘next generation’ omicron booster vaccine in world first
Joe Pinkstone – 29m ago
The Telegraph
https://www.msn.com/en-gb/news/uknews/uk-authorises-next-generation-omicron-booster-vaccine-in-world-first/ar-AA10FJ3i?ocid=msedgdhp&pc=U531&cvid=40f6ba5663e4444ab4ae036b374197c5
‘Sharpened tool in our armoury’
Dr June Raine, the chief executive of the MHRA, said: “I am pleased to announce the approval of the Moderna bivalent booster vaccine, which was found in the clinical trial to provide a strong immune response against the omicron BA.1 variant as well as the original 2020 strain.
“The first generation of Covid-19 vaccines being used in the UK continue to provide important protection against the disease and save lives. What this bivalent vaccine gives us is a sharpened tool in our armoury to help protect us against this disease as the virus continues to evolve.
“We have in place a comprehensive safety surveillance strategy for monitoring the safety of all UK-approved Covid-19 vaccines and this will include the vaccine approved today.”
GOV.UK
Navigation menu MenuSearch GOV.UK
Home
Marketing authorisations, variations and licensing guidance
Decision
Regulatory approval of Spikevax bivalent Original/Omicron booster vaccine
Information for healthcare professionals and the public on Moderna’s bivalent vaccine. Information on the original Spikevax COVID-19 vaccine can found on a separate page (link below)
From:
Medicines and Healthcare products Regulatory Agency
Published
15 August 2022
Get emails about this page
Documents
Summary of Product Characteristics Spikevax bivalent Original/Omicron
PDF, 476 KB, 21 pages
Patient Information Leaflet Spikevax bivalent Original/Omicron
PDF, 245 KB, 8 pages
Spikevax bivalent Original/Omicron Information for Healthcare Professionals (Regulation 174)
PDF, 476 KB, 23 pages
Details
The 15-minute observation period following vaccination with the mRNA COVID-19 vaccines has been removed for individuals aged 12 years and over who have no history of a severe allergic reaction (as outlined in the Greenbook advice This follows careful review of the safety data by the MHRA and advice from the government’s independent Commission on Human Medicines. A temporary suspension of the 15-minute observation period for children aged 5-11 years remains in place and this will be reviewed on a regular basis.
The product information for the Spikevax original COVID-19 vaccine (formerly COVID-19 Vaccine Moderna) can be found on a separate page.
The MHRA has issued a Conditional Marketing Authorisation for Spikevax bivalent Original/Omicron booster vaccine in Great Britain and a temporary Regulation 174 authorisation for Northern Ireland to ensure supply across all of the UK. If the European Medicines Agency grants a CMA for Spikevax bivalent Original/Omicron booster vaccine it would apply in Northern Ireland and the Regulation 174 authorisation would no longer be in place.
Supply of this product will be subject to the same requirements in Great Britain and Northern Ireland.
The information for healthcare professionals and UK recipients on using the bivalent vaccine safely will be periodically updated as new data become available and this will continue when the CMA is converted to a MA. Please regularly check this information as it is often updated.
The Public Assessment Report will be published shortly.
Summary of Product Characteristics (also referred to as the Information for Healthcare Professionals under R174)
This is a description of a medicinal product’s properties and the conditions attached to its use. It explains how to use and prescribe a medicine. It is used by healthcare professionals, such as doctors, nurses and pharmacists.
Patient Information Leaflet
The Patient Information Leaflet provides information for patients on using the medicine safely. This is based on the Summary of Product Characteristics of the product.
Public Assessment Report
The Public Assessment Report is a scientific report, written by the MHRA. It explains how this product was assessed and its authorisation recommended, as well as its conditions of use. It is not intended to provide practical advice on how to use this product.
Published 15 August 2022
Get emails about this page
More than Ambivalent about bivalent Spikevax Seems more important to MHRA to be the first to approve it – make it ‘conditional’ and backs are covered. Remember they have made it the publics’ responsibility to look out for serious adverse effects.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you are given Spikevax bivalent Original / Omicron if:
– you have previously had a severe, life-threatening allergic reaction after any other vaccine
injection or after you were given Spikevax or Spikevax bivalent Original / Omicron in the past.
– you have a very weak or compromised immune system
– you have ever fainted following any needle injection.
– you have a bleeding disorder
– you have a high fever or severe infection; however, you can have your vaccination if you have a
mild fever or upper airway infection like a cold
– you have any serious illness
– if you have anxiety related to injections
Myocarditis/pericarditis
There is an increased risk of myocarditis (inflammation of the heart muscle) and pericarditis
(inflammation of the lining outside the heart) after vaccination with Spikevax bivalent Original /
Omicron (see section 4).
These conditions can develop within just a few days after vaccination and have primarily occurred
within 14 days. They have been observed more often after the second dose of Spikevax (original), and
more often in younger males.
Following vaccination, you should be alert to signs of myocarditis and pericarditis, such as
breathlessness, palpitations and chest pain, and seek immediate medical attention should these occur.
If any of the above apply to you (or you are not sure), talk to your doctor, pharmacist or
nurse before you are given Spikevax bivalent Original / Omicron.
Capillary leak syndrome (CLS) flare-ups
A few cases of capillary leak syndrome flare-ups (causing fluid leakage from small blood vessels
(capillaries) resulting in rapid swelling of the arms and legs, sudden weight gain and feeling faint, low
blood pressure) have been reported following vaccination with Spikevax. If you have previously had
episodes of CLS, talk to a doctor before you are given Spikevax bivalent Original / Omicron.
Duration of protection
As with any vaccine, a booster dose of Spikevax bivalent Orginal / Omicron may not fully protect all
those who receive it and it is not known how long you will be protected.
Children
Spikevax bivalent Original / Omicron is not recommended for children aged under 18 years.
Other medicines and Spikevax bivalent Original / Omicron
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other
medicines. Spikevax bivalent Orginal / Omicron may affect the way other medicines work, and other
medicines may affect how Spikevax bivalent Orginal / Omicron works.
Immunocompromised individuals
A booster dose of Spikevax bivalent Orginal / Omicron may not provide full immunity to COVID-19
in people who are immunocompromised, and you should continue to maintain physical precautions to
help prevent COVID-19. In addition, your close contacts should be vaccinated as appropriate. Discuss
appropriate individual recommendations with your doctor.
Pregnancy and breast-feeding
Spikevax bivalent Original / Omicron can be used during pregnancy. A large amount of information
from pregnant women vaccinated with Spikevax during the second and third trimester has not shown
negative effects on the pregnancy or the newborn baby. While information on effects on pregnancy or
the newborn baby after vaccination during the first trimester is limited, no change to the risk for
miscarriage has been seen.
3
Spikevax bivalent Original / Omicron can be given during breastfeeding. A large amount of
information from breastfeeding women vaccinated with Spikevax has not shown negative effects in
breastfed babies.
If you are pregnant or think you may be pregnant, and have any questions or concerns, tell your
doctor, nurse or pharmacist before you receive this vaccine.
Driving and using machines
Do not drive or use machines if you are feeling unwell after vaccination. Wait until any effects of the
vaccine have worn off before you drive or use machines.
Spikevax contains sodium
Spikevax bivalent Original / Omicron contains less than 1 mmol (23 mg) sodium per dose and, that is
to say, essentially ‘sodium-free’.
3. How you will be given Spikevax bivalent Original / Omicron
Individuals 18 years of age and older
A booster dose will be given to you as a single 0.5 mL (50 microgram) injection. This should be at
least 3 months after a second dose or a booster dose of a COVID-19 vaccine.
Your doctor, pharmacist or nurse will inject the vaccine into a muscle (intramuscular injection) in your
upper arm.
During and after each injection of the vaccine, your doctor, pharmacist or nurse will watch over you
for at least 15 minutes to monitor for signs of an allergic reaction.
If you have any further questions on the use of this vaccine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them. Most side
effects go away within a few days of appearing. If side effects such as pain and/or fever are
troublesome, they can be treated by medicines for pain and fever such as paracetamol.
Get urgent medical attention if you get any of the following signs and symptoms of an allergic
reaction:
– feeling faint or light-headed;
– changes in your heartbeat;
– shortness of breath;
– wheezing;
– swelling of your lips, face, tongue or throat;
– hives or rash;
– nausea or vomiting;
– stomach pain.
Talk to your doctor or nurse if you develop any other side effects. These can include:
Very common (may affect more than 1 in 10 people):
– swelling/tenderness of the underarm glands
– headache
– nausea
– vomiting
– muscle ache, joint aches, and stiffness
4
– pain or swelling at the injection site
– redness at the injection site (some of which may occur approximately 9 to 11 days after the
injection)
– feeling very tired
– chills
– fever
Common (may affect up to 1 in 10 people):
– diarrhoea
– rash
– rash or hives at the injection site (some of which may occur approximately 9 to 11 days after the
injection)
Uncommon (may affect up to 1 in 100 people):
– itchiness at the injection site
– dizziness
– stomach pain
Rare (may affect up to 1 in 1,000 people)
– temporary one-sided facial drooping (Bell’s palsy)
– swelling of the face (Swelling of the face may occur in patients who have had facial cosmetic
injections.)
– decreased sense of touch or sensation
– unusual feeling in the skin, such as tingling or a crawling feeling (paraesthesia)
Very rare (may affect up to 1 in 10,000 people)
– inflammation of the heart muscle (myocarditis) or inflammation of the lining outside the heart
(pericarditis) which can result in breathlessness, palpitations or chest pain
Frequency unknown
– severe allergic reactions with breathing difficulties (anaphylaxis)
– reaction of increased sensitivity or intolerance by the immune system (hypersensitivity)
– a skin reaction that causes red spots or patches on the skin that may look like a target or
“bulls-eye” with a dark red centre surrounded by paler red rings (erythema multiforme)
PS
More than Ambivalent about bivalent Spikevax Seems more important to MHRA to be the first to approve it – so made it ‘conditional’ and backs are covered. Remember they have made it the publics’ responsibility to look out for serious adverse effects.
check under Warnings and precautions
“We’re going to start rolling …..
https://www.telegraph.co.uk/news/2022/08/16/timing-covid-booster-important-type-insists-deputy-jcvi-chair/
‘Timeliness much more important than vaccine type’
Speaking to the BBC, Professor Harnden said: “The timeliness is much more important than the type of vaccination we believe, though we do welcome this bivalent vaccine approved by the MHRA.
“At the moment, we’re saying get vaccinated and don’t worry too much about the type of vaccine.
“There may well be other vaccines in the pipeline – Pfizer I believe are developing a bivalent vaccine which we’ll look at very carefully on JCVI if its approved.
“And of course, the Government may order, or may have ordered, some more bivalent Moderna vaccines so that there’s going to be a suite of vaccines which are available to use.”
Alan Bishop7 MIN AGO
Yet another ‘getcha covid jab’, so that big pharma can continue making lotsa lolly from the gullible punters.
Yet another jab that will neither stop you catching the pox or passing it on. (The very opposite to the true definition of a vaccine).
I’m 75, am Type 2 diabetic and also have a damaged kidney- but I have never had any covid jab and no intention of having one.
“For simplicities sake ….. “
Today – Radio 4
https://www.bbc.co.uk/sounds/play/m001b40p
1.31.40
“What about AstraZeneca”………………. ‘the beauty’ …
Maybe a dose of Spikevax would help poor Albert
@AlbertBourla
·
15 Aug
I would like to let you know that I have tested positive for #COVID19. I am thankful to have received four doses of the Pfizer-BioNTech vaccine, and I am feeling well while experiencing very mild symptoms. I am isolating and have started a course of Paxlovid.
Albert Bourla
@AlbertBourla
We have come so far in our efforts to battle this disease that I am confident I will have a speedy recovery. I am incredibly grateful for the tireless efforts of my Pfizer colleagues who worked to make vaccines and treatments available for me and people around the world.
12:52 pm · 15 Aug 2022·Twitter Web App
@AlbertBourla
·
15 Aug
Replying to
@AlbertBourla
Paxlovid is not approved, but is authorized for emergency use by the FDA to treat mild-to-moderate COVID-19 in high-risk patients 12+, weighing at least 40 kg, with positive results of SARS-CoV-2 viral testing. See safety info: http://COVID19oralRx.com.
The chickens are coming home to roost for Pfizer
16th August 2022 by Nadya Swart
https://www.biznews.com/health/2022/08/16/chickens-home-roost-pfizer
Having succeeded in foisting Covid-19 mRNA injections on an initially unsuspecting public, it appears that the chickens have come home to roost for Pfizer. Pfizer’s criminal withholding of vaccine data is particularly reprehensible when it comes to those children who received a Covid-19 vaccine on the basis that it was not only completely safe, but necessary. A study in Thailand conducted during the country’s national Covid-19 vaccination campaign for adolescents showed what one physician described as a “stunning” association between myocarditis and the Pfizer-BioNTech vaccine. Studies of this nature are emerging across the globe, with the most damning evidence of the vaccine’s dangers so far being the report that came from the pharmaceutical behemoth itself. This article was first published on the Daily Friend. – Nadya Swart
Pfizer gives damning evidence against Pfizer vaccines
By Andrew Kenny*
The most damning report I have ever seen on the dangers of Covid-19 vaccines comes from Pfizer Incorporated.
In it, Pfizer lists the adverse events shortly following its own vaccination, including death, heart damage, disorders of the gastric system and nervous systems, and of the skin and eyes.
What makes the report more damning is the fact that Pfizer and the US Food and Drug Administration (FDA) tried to suppress it until a US court of law forced them to make it public.
Maryanne Demasi, PhD
@MaryanneDemasi
·
Aug 16
The FDA’s evidentiary standards for drug approvals has significantly declined. Faster approvals based on fewer, smaller & less rigorous trials has led to distrust, not only of the agency, but in the safety & effectiveness of medicines in general
https://maryannedemasi.substack.com/p/the-declining-standards-of-fda-drug
The declining standards of FDA drug approvals
Maryanne Demasi, PhD
https://maryannedemasi.substack.com/p/the-declining-standards-of-fda-drug
The US Food and Drug Administration (FDA) has a legal obligation to protect the public and ensure that the benefits of medicines outweigh the harms before being marketed to people.
But the agency’s increasing reliance on pharmaceutical industry money has seen the FDA’s evidentiary standards for drug approvals significantly decline.
Independent experts now say the declining evidentiary standards, shortening approval times, and increasing industry involvement in FDA decision-making, has led to distrust, not only of the agency, but in the safety and effectiveness of medicines, in general.
Pfailing and Pflailing…
Re: Covid-19: Study provides further evidence that mRNA vaccines are safe in pregnancy Jacqui Wise. 378:doi 10.1136/bmj.o2013
Dear Editor
Further to my recent response, it would appear the UK Government are now saying singing a different tune.
Under the heading “Toxicity conclusions.”
“In the context of supply under Regulation 174, it is considered that sufficient reassurance of safe use of the vaccine in pregnant women cannot be provided at the present time: however, use in women of childbearing potential could be supported provided healthcare professionals are advised to rule out known or suspected pregnancy prior to vaccination. Women who are breastfeeding should also not be vaccinated.”
https://www.gov.uk/government/publications/regulatory-approval-of-pfizer…
Douglas R Hendrie
Hi
About the coding tricks for Jaundice and the RSV vax, I suppose that the goal is to split all the events between 3 different SOCs.
About the statistical significance, if you use a Poisson distribution all of these RR are significant 🙂