This is the first of 3 linked lectures given to family doctors in Stockholm on September 9 2021. The meeting was organized by André Marx, a family doctor in Stockholm who also runs Sweden’s first and only public health care-funded withdrawal clinic for psychiatric drugs.
The alternate title of this talk is Girl with a Clinical Trial Tattoo. It will be followed by Girl who Catches Ghosts and Girl who Eats Salt. All will feature on the Politics of Care Forum.
Thomas Hultgren who works in Equal – a patient organization in Stockholm – made a video recording with occasional Zoom effects – children talking. The Video is HERE.
Slide 1
I come from a very orthodox, conservative medical background. I believe in the medical model and the value of pharmacological and other medical treatments. Despite this you may be disturbed by what you hear. Disturbed by the distance we seem to have travelled from what They Used to Call Medicine to what is happening now.
I won’t be offering many answers. But I have two questions – What are RCTs and What is Data. My answer to Data is in this talk. My answer to RCTs is in the third talk.
Slide 2
Here is our patient. It’s not clear if it is a woman or a man. I can tell you s/he is smelly. You might initially feel s/he is not very bright. How do we Help here? What would Help look like? Help surely cannot be the same as telling someone what to do.
Slide 3
Our problems knowing what to do for our patient start here. Any discussion of randomized controlled trials (RCTs) starts with Ronald Fisher, a cantankerous character. Fisher thought the idea that smoking causes lung cancer was ridiculous – views the tobacco industry were happy to fund. Fisher wasn’t a doctor or a scientist. He never ran an RCT. He was a mathematician who was interested to mathematically characterise the views of experts.
Fisher assumed experts knew what they were doing. For him experiments were a way to demonstrate that – not a way to find out new things. Only two things could get in the way of an experiment turning out the way the expert said.
Slide 4
There might be some unknown factor, but Fisher thought this would be rare – experts knew nearly everything. Randomization could take care of unknown unknowns.
Fisher’s experts were like Robin Hood. Unless chance intervenes, like Robin Hood they would split the first arrow 19 times out of 20.
Slide 5
If you can any medical condition where the RCTs turn out like Fisher’s scenario – let me know. Trials of antidepressants and most drugs look more like this – all over the place.
If medical experts were like Fisher thought, you wouldn’t need trials. Medical advice would be like telling people to wear a parachute when jumping out of a plane.
Slide 6
This old Deutschmark celebrates Carl Friedrich Gauss’ key scientific breakthrough. Around 1810 telescopes were unreliable and astronomers couldn’t be sure if they were looking at one or two stars. Gauss solved this with the confidence interval which you see beside him – if the measurements fell within the confidence interval they were from the same star and from different stars if one of them fell outside it.
Jerzy Neyman and Egon Pearson, who hated Fisher, said statistical significance was all wrong and experiments needed confidence intervals.
These guys also had nothing to do with science or medicine. They never did an RCT. But in the 1970s, medical journals told us to drop statistical significance and start using confidence intervals instead.
Slide 7
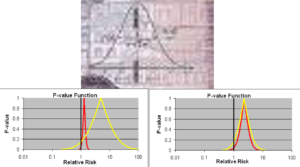
This is the only complicated slide in this talk. You see Gauss’s confidence interval on top. Confidence intervals work for a measurement error problem like stars and telescopes. If you give a beta blocker to one person, their heart rate will slow but with some variation every time you check. We can call this measurement error and use Confidence intervals – the data will all be to the Left of the heavy line running through 1.0.
But if you give a beta blocker to ten people, it will slow the heart rate of 9 but the next person may have a heart rate increase. We should but we don’t view her as a different star. We just say beta-blockers slow heart rate.
Heart rate increases on beta blockers are rare but many psychotropic drugs sedate some while leaving others wide awake. Half of the measurements will fall to the Left of 1.0 and half to the Right, leaving companies able to claim their drug has no effect on sleep. This is not just wrong its psychotic.
On the lower Left we have another example. Both the Red and Yellow Drug here can kill you. If forced to take one of them, all your statistical training will tell you avoid the Red one because the confidence interval says it can kill you for sure. The Yellow confidence interval crosses 1.0 so supposedly we don’t know if it can kill you.
Well – the likely risk from the Yellow Drug is nearly 10 times greater than the Red one. If forced to choose, you should take the Red one.
The next example shows the suicidal events from FDA’s database of antidepressant trials – the Red Curve shows events in those 25 and under and Yellow Curve people who are 45-55. But FDA and others claim only those 25 and under are at risk from antidepressants. There’s no problem if you are over that age.
The bottom line here is that neither statistical significance nor confidence intervals work in trials of a medicine. We have tens of thousands of RCTs now but no-one can really work out what they mean. Our use of statistics is based on convenience rather than reality. Confidence intervals can work if we know what we are doing in the first place but don’t if we don’t know what we are doing. See The Antidepressant Tale: Figures Signifying Nothing.
Slide 8
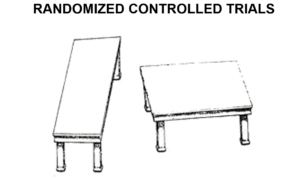
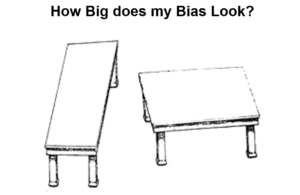
The two tables you see here are identical in size and shape. If you superimpose one on the other, they will match – confounding you. Randomizaton supposedly controls for unknown confounders. People talk about it in mystical terms. Put a drug through an RCT and even though a doctor has no idea what they are doing – the right answer will emerge.
The problem with this is neither Fisher nor Neyman ever thought an RCT could help someone who didn’t know what they were doing. You have to roughly know what you are doing before an RCT can be of any use.
Slide 10
The First Medical RCT was for streptomycin in tuberculosis in 1948. Tony Hill was the person who introduced randomization to a medical trial. Hill didn’t follow Fisher or Neyman. There was nothing mystical about randomization in his trial – it was just a method for fair allocation.
Two years previously clinicians at the Mayo Clinic had done an old-fashioned trial to evaluate streptomycin for tuberculosis – controlling for things like age and sex in both treatment and placebo groups. Both trials found streptomycin worked. The Mayo trial found patients became resistant quickly and some went deaf. Hill’s RCT missed all this.
The Mayo studies were the only reason Hill’s trial was run – the answer to did streptomycin work was already known. Hill didn’t discover it.
Slide 10
After Hill’s RCT, very few investigators could see much point in doing RCTs rather than the usual kind of clinical trial. Something else happened to change that – as you will see.
Here’s Tony Hill in 1965 reflecting on RCTs. In an early part of this article, he says he is surprised at how popular RCTs have become. He is also surprised by the fact its mostly industry people pushing them – not doctors.
He says RCTs are just one way to evaluate Therapeutic Efficacy – that is evaluating one of the 100 or more effects all drugs have. This essentially means RCTs are not a good way to evaluate a drug – they have a place but do not offer a good view of a drug overall.
Slide 11
In the 1950s the most enthusiastic advocate for RCTs was Louis Lasagna. Lasagna had put the placebo and Clinical Pharmacology on the map.
Drug Regulation then was about Drug Safety. Lasagna thought RCTs offered FDA a way to establish if a drug worked. If they didn’t work, they couldn’t be safe. Nobody paid any heed.
Slide 12
Events changed everything. The horrific birth defects caused by Thalidomide triggered a political crisis – something had to be done.
The 1938 FDA Act which focused on safety kept Thalidomide off the US market. A new 1962 Act gave FDA a brief to establish that drugs were effective in addition to safe. Demonstrating effectiveness would be done using RCTs. The idea was 2 positive RCTs would be the criterion. Everyone thought if there was one positive trial, all trials would be positive, and certainly with two positive trials – but we now know this is not the case. 50% of trials can be negative.
Adding effectiveness can only be a good thing – don’t you think? Well thalidomide later got licensed – under the 1962 Effectiveness Act.
Slide 13
Prior to the 1962 Act, only one drug had been shown to be both safe and effective in a placebo controlled RCT – Thalidomide and the person who did the trial was Louis Lasagna. Article Here. The mechanism we have put in place to stop Thalidomide happening again was one that it sailed through without a problem.
Everyone thought, and most still think, RCTs put a brake on pharmaceutical companies. Instead, RCTs have become the standard through which companies make Gold.
Slide 14
Twenty years later you see Lasagna responding to Rossi et al who say RCTs are the most sophisticated way to work out what a drug is doing.
He says this is only true if sophisticated means adulterated. This is an older meaning of the English word sophisticated that most people today don’t realise. Sophisticating wine means adding ethylene glycol to it. Article Here.
He is saying essentially that clinical judgment is more accurate than RCTs at least for adverse events and more interesting to engage with.
Slide 15
Ten years later again you have him saying that his view of RCTs has changed completely since the 1950s. Interview Here.
And RCTs aren’t that useful – certainly not for the key question which is what am I going to do to help this person in our waiting room.
Slide 16
RCTs are supposed to control all confounders even the ones we don’t know about. In fact, it is just the opposite. RCTs introduce confounders and make trial results essentially meaningless.
Slide 17
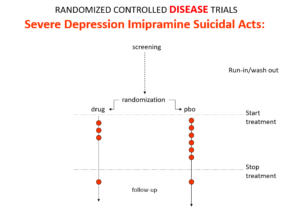
Imipramine was the first antidepressant. It and other tricyclic antidepressants are stronger than SSRIs and SNRIs. It beats them in RCTs. It can treat melancholia – they can’t. They are useless for severe depression. Melancholia comes with a high risk of suicide.
Imipramine was launched in 1958. A year later at a meeting in England, Danish psychiatrists made it clear that while it was a wonderful treatment it made some people suicidal. Nobody there argued. This drug can cause suicide.
Let’s do a thought RCT of imipramine versus placebo in melancholia. Even though it can cause suicide, we would expect it to reduce the number of suicides in a trial like this because it treats the condition. This RCT would be great evidence antidepressants do not cause suicide.
Slide 18
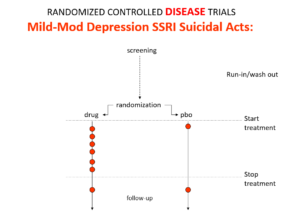
Here is the data on the trials in mild depression that brought the SSRIs and SNRIs on the market – you see a doubling of suicidal events compared to placebo. Companies resorted to all sorts of illegal manoeuvres to hide this risk.
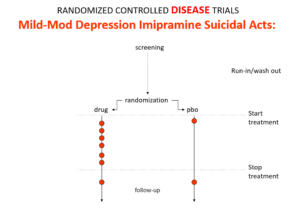
Slide 19
This is what the data for imipramine look like in the same mild depressions. Now it seems that it too causes suicides. So RCTs tell us nothing about cause and effect – they can give us diametrically opposite answers. This is because these aren’t drug trials. They are Treatment Trials and in any clinical Trial, the condition confounds the effects of the drugs – and these confounders hide drug effects.
People evaluating drugs in traditional trials, before RCTs, knew this. People doing RCTs don’t. When a patient becomes suicidal in a trial you have to use your judgement to work out what is happened but in RCTs clinicians are not supposed to use their judgment.
Slide 20
This is not just the case for depression – it’s true in every clinical situation where drugs and conditions cause superficially similar effects – diabetes and glitazones both cause heart failure, osteoporosis and bisphosphonates both cause fractures
Slide 21
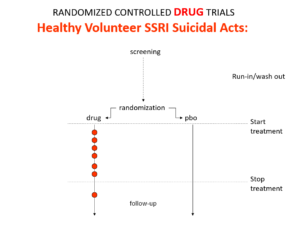
Here is what a drug trial looks like. Companies ran these studies in the 1980s and found that SSRIs make healthy volunteers suicidal, caused dependence and sexual dysfunction but we heard nothing about these problems when the drugs launched. These Drug Trials enabled companies to engineer their Treatment Trials to hide these problems. I will show you how this is done now in a moment but first look at this.
Slide 22
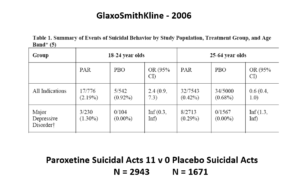
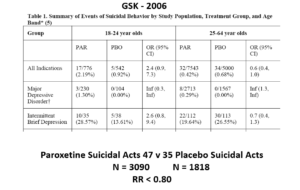
This slide shows some data straight from a 2006 GlaxoSmithKline paper. GSK’s SSRI paroxetine was in trouble – the RCTs data for Major Depressive Disorder seem to show paroxetine causes suicidal events. The real data I think are worse that GSK admit to here.
Slide 23
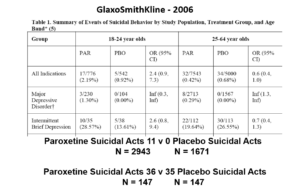
But never fear RCTs come to the rescue. GSK also did trials in people with Intermittent Brief Depressive Disorders – IBDD. These are borderline personality disorder to most people – patients who have suicidal events much more often than anyone else. But these patients can meet criteria for depression and could be entered into Depression RCTs.
Now Lilly had done a trial of Prozac in these patients – it didn’t work. GSK also did a trial of paroxetine which didn’t work and had a 3-fold higher suicidal act rate than placebo. GSK then did another trial in a similar group of patients. Why?
The answer is here. Here are IBDD data from the two GSK trials. I have seen other data for these two trials which make paroxetine look worse but let’s stick with GSK’s story. We could even add 16 more events to the paroxetine arm and still get the same magical outcome
Slide 24
When you add the IBDD data to the MDD data – all of a sudden paroxetine doesn’t cause suicidal events, it protects against them.
Something like this is going to happen in every treatment trial where the patients entered are heterogenous – back pain, breast cancer, diabetes, hypertension, osteoporosis, parkinson’s disease. We can use an effect a drug causes to hide an effect a drug causes.
RCTs are not a good way to work out what is going on. Results of a back pain trial will insist you use analgesics rather than antibiotics – which is all wrong for the 5-10% of backpains caused by infections.
Slide 25
Now I mentioned in Healthy Volunteer Drug Trials, companies saw SSRIs give most people sexual problems. After only 2 weeks, you can be left unable to function ever again in your life – the condition is called Post SSRI Sexual Dysfunction (PSSD). It happens in young and old, female and male, all ethnic groups and in every country on earth.
But in company Treatment Trials, less than 5% of people seem affected in this way.
No laws need to be broken to achieve this. No skullduggery is needed. Just do an RCT. RCTs depend, as Tony Hill told you, on a primary endpoint. Everybody assumes this is the commonest thing a drug does, and devoting attention to it makes sense. All other effects will be less common or may only show up if you’ve been on treatment for months or years.
For SSRI RCTs, the primary endpoint is mood change. But mood change is not the most common effect. It is the effect of commercial interest. What happens if you focus all attention on this –
Slide 26
This is what happens…. We are hypnotized and miss what is going on
Slide 27
The sexual effects of SSRIs happen in close to 100% of takers within 30 minutes of the first dose. They should be unmissable. But a focus on a primary endpoint makes them vanish.
Slide 28
Companies also knew from healthy volunteer trials that people become dependent on SSRIs but this problem vanished in RCTs. The result is that 10-15% of the population of most Western countries are now on these drugs – primarily because they can’t get off.
The BMJ ran a lead article 2 years ago saying the British have stopped making love. They blamed depression but those of us in the mild states these drugs get given for often turn to love-making or eating to help. The Benzodiazepines we used for these problems before the SSRIs didn’t cause any worse dependence and we were at least able to make love.
It’s the drugs 15% of us are on that make love-making impossible for maybe 20% of the population if you take our partners into account and perhaps 30% of people in areas where the use of these drugs is particularly high.
Slide 29
RCTs greenwash drugs. They convert poisons out of which we can bring good, if we are not hypnotized, into sacraments. Sacraments are substances that can only benefit – that cannot harm.
Regulators tell us that drugs that kill us or wipe out our sex lives for ever have a positive benefit-risk balance. This claim is based on RCTs, which only look at one of a drug’s effects – that may not be the most common effect. The statement is totally incoherent – it was a drug company invented mantra that regulators have swallowed.
Slide 30
One of my key questions at the start of this talk was – What is Data?
In a trial of Pfizer’s antipsychotic, a man died from burns. You can’t get any sense of what happened him from reading any of the 50 articles there are from every single drug trial. Few mention any hazards. The figures and statistical outputs in the papers from this trial give no hint.
His death triggered an internal company adverse event report. This shows he poured petrol on himself and set fire to it in an attempt to kill himself. He died 5 days later – and was coded as death by burns. If you didn’t have the adverse event report – which you can’t easily get – you’d have no way know what happened.
Slide 31
In GSK’s famous Study 329,which you will hear more about in the second lecture, a trial of paroxetine versus placebo in teenage depression, a 15-year-old boy was arrested by police because he was out on the street with a gun threatening to shoot people. The police took him to hospital. He was taking paroxetine. This should have led to a serious event report but it didn’t because GSK had discovered if you say someone has an intercurrent illness – you don’t have to report on what happened. Four children dropped out of this trial with intercurrent illness – all on paroxetine.
A internal company email, which FDA never got to see, told the story of this boy. He brings out the meaning of data.
People are the data in clinical trials. You have to be able to interview them to find out if this boy’s case if he had an adverse event or did he really have another illness – did it clear when the drug was stopped?
Working out has a drug caused a problem is judicial – you do it by examination and cross-examination not by counting out the figures an algorithm spews out. What Used to be Called Medicine was judicial – it was not algorithmic.
Slide 32
Imagine you break an arm and go to an Accident Department who say ‘Good news we are running an RCT of Plaster of Paris. We are going to put a POP randomly on one of your 4 limbs and compare this to no POP’.
Randomly putting a POP on some limb will do better than no POP but to start doing this on the basis of RCT results like this would be crazy. This however is exactly what we are doing.
Slide 33
This however is what most doctors are doing. RCTs effectively remove our brains and replace them with something that can be programmed.
Slide 34
RCTs are pitched against clinical judgment. Clinical expertise used to be at the heart of HealthCare – lived experience we could call it – but this is now a problem for health service companies.
Traditional doctors are like a gourmet Chef commenting about a Fast-Food meal and health has become a service industry like Fast Food – gourmet physicians aren’t wanted.
We are a problem for Guideline writers, regulators, and pharmaceutical companies, all of whom want to pitch objective knowledge in the form of RCTs against your and my expertise. The idea of bringing good out of the use of a poison doesn’t compute for insurers, managers or even the public who want religious sacraments.
What are these RCTs that are used to invalidate us? The answer comes later.
The Politics of this are that in some sense we need health/medical co-operatives rather than Corporate Operations in which staff and patients are Cogs rather than central.



















Thank you for the blog and for the the videos . In a nutshell they will find anyway of squirting poisons into our bodies, presently focussing on vaccines obviously,
including those of very young children without any real evidence of either protecting them, or as some ‘scientists; medics; politicians; pharma companies are claiming, that it is ethical. moral and good medicince to be using children as a shield to protect infection from being spread in the wider community, Vaccination of children 12yrs to 15yrs has started in the UK today. They are considered able to decide for themselves whether to have the vaccine regardless of what their parents/guardians decide is best for them. Any disagreement between child and adult is to be decided by – a doctor. If only doctors could be relied on to do their best for us rather than bow to authorities who also cannot be trusted
“My favourite quotation was taken from Austin Bradford Hill’s Heberden Lecture in 1965: “If one came to the conclusion that the only way to find out the truth about a medication was to use a controlled clinical trial, it would mean not that the pendulum had swung too far but that it had come completely off its hook”. “In another part of the paper [Hill] comes up with something else that I have been trying to sell to people with not an awful lot of luck. What he said was that a controlled trial does not tell the physician what he would like to know which is how do I know in advance without engaging in trial and error which antidepressant is better for Mr Jones or Mrs Smith. That’s what doctors and patients would like to know. We don’t do that”.
Data Transparency does not address the problem of the use of recruited patients. It doesn’t necessarily address the problem of statistical versus clinically relevant efficacy. But Dr. Healy is after bigger game than simply the misuse of scientific analysis in clinical trials. He is questioning the whole of elevation of the clinical trial to the position of the final word in medicine. We’ll be hearing more from him about that as his series progresses.
What I would say about all of this is simple: of course the clinical trial data is not the gold standard – the patient in front of me wasn’t in it. I would say the same thing about the deification of the DSM diagnostic categories – my patient wasn’t in the field trials either. Those things are in “the cloud” along with everything else I’ve ever learned along the way, and everything the patient has learned along the way. The clinical issue in our meeting is to come up with the best path for the patient with the time and resources at hand. Accurate clinical trials are sometimes a big help, but clinical trial results hardly make clinical decisions or define a clinician.
The scheme below might make an iPhone App, but it’s not clinical medicine:
http://1boringoldman.com/index.php/2013/04/09/not-clinical-medicine/
Some years ago I went to a mental hospital where here in Brazil so that I could take the receipts to buy the legal drugs they decided I needed.
That day I sat next to a woman and I started talking to her.
She said she was diagnosed OCD and was “taking the drugs from the research”.
Fortunately she was beside me And couldn’t see my eyes that popped out in horror.
Note she said “the research”.
I smiled at her and asked her how was that. She opened a laminated parcel that had no name, only numbers.
She didn’t know she was being part of a clinical trial. It was only “a research”.
😔
Remember Traci Johnson, a 19 years old woman who hanged herself in Elli-Lilly’s facilities back in 2007 during Cymbalta trial.
http://hellaheaven-ana.blogspot.com/2010/01/post-from-my-other-blog-february-7.html?m=1
This is how I described one of my suicide ideation:
Psychiatric drug-induced suicide attempt: how to differentiate real suicide from drug-induced (repost)
https://justana-justana.blogspot.com/2012/01/psychiatric-drug-induced-suicide.html?m=0
There is a huge difference between wanting to die and just the act of killing oneself that is planted in the mind when we are dealing with drug-induced suicidal ideation. Trust your instincts and, please, search for help if you feel you are suicidal because of an antidepressant or any other drug.
This is my experience and I only published to make others understand that drug-induced suicidal ideation is REAL!I didn’t write about the second because it is too hard.
“One of the strange feelings when someone or something do you harm is the mixture of feelings you have towards yourself. You feel as if it was your fault and you feel ashamed to tell others what has happened. Of course there is anger towards what did you harm but it’s usual that people don’t tell others about it.
We remain silent and hoping that someone else suffers the same and have the guts to tell others.
I said that I had suicidal ideation while tapering Effexor. What I didn’t say is that I’ve tried to kill myself twice. I thought about it on a wide scale of degrees. Four times it was very hard to cope with it and for two times I’ve tried.
I’ll tell you about one of these times.
I was in a normal day, tapering Effexor. All of a sudden, an idea was planted in my brain: “-I have to kill myself.” Just like that. Unexpectedly, no reason for it, I was happy and then this idea appeared.
You don’t think about anything else. You only think that you have to kill yourself. I wrote some notes for four people, and was thinking at the back of my mind: “-This is withdrawal, this is withdrawal, this is withdrawal…; call your therapist, call a friend, do something!”
Strangely enough you don’t call anybody. You do not care. All you have to do is… kill yourself.
I have a dog. So I could not do anything at home for I could not harm her or make something that could kill her, like gas – my second attempt was with gas -, and you keep on wandering how are you going to do it without making any fuss and avoiding the scandal of being found dead in your place. Good, at least there’s room to think about a dignified exit!
I had many samples of psychiatric drugs, drugs that I tried, and, at the forth pill had to stop… I had an arsenal of psychiatric drugs of many kinds.
Therefore, I took them all and put them in two bottles of Depakote – by that time it was sold in bottles not in blister. “-It’s withdrawal, it’s withdrawal, it’s withdrawal… do something; call someone; call your therapist, please!” “-Nope! I have to kill myself.”
I’ve phoned a hotel and ask for a bedroom. I’ve dressed myself with care and took a big bag pretending to be coming from a near town. I have put some clothes in this bag and a bottle of Jack Daniels to have the pills, Rohypnol was in the cocktail which is very helpful and was once used by the site Exit . They used to sell a packed for those who wanted to do euthanasia and I’ve discovered that one of the three items was Rohypnol. They are back now but with another proposal.
“-It’s withdrawal, it’s withdrawal, it’s withdrawal… do something; call someone; call your therapist, please!” “-Nope! I have to kill myself.”
It was 9 pm. I went away from my building, took a cab, and told the driver to go to the hotel. He left me there.
When I was in front of the hotel, I felt thirsty and did not want to appear as if I was out of my mind. I went to a place and asked for a bottle of water.
I thought that the man could not hear me. By miracle, he gave me the bottle of water. I took it and, miracle, I’ve paid for this and he smiled at me. He smiled at me!
So people could see me! “-It’s withdrawal, it’s withdrawal, it’s withdrawal… do something; call someone; call your therapist, please…
Isn’t it good!
I’m alive! I started walking. I’ve walked, walked, walked, and started to sweat.
Nice feeling! I was sweating and feeling all my body, my legs, my arms, my head, my hands, my toes…
“-It’s withdrawal, it’s withdrawal, it’s withdrawal…”
What am I doing here? Why will I kill myself? I don’t want to kill myself.
My dog is home! She must be feeling sad. I have to go back home to see her and call my friends and family.”
“I want to thank Charles Medawar, SocialAudit. There was a man on his site whose nick was “Anon”. He helped everybody and one of the things I’ve remembered was he saying that we should never become a statistics and if we killed ourselves “they” were winning another time.
He said other valuable things that was on my mind beside the “-It’s withdrawal…”
Fortunately I don’t remember anymore and I’m glad to be able to talk about it without crying and now I am feeling that it’s in the past.
The only thing I fear is that even spending 19 months tapering Efexor when I reached the end of the process I felt so bad that I had to go back to the drug.
I’ll talk about it later.
If I miss I pill I have nightmares. I fear missing the amount of dose and feel it again.
You can see that it’s very easy to kill me if someone has the intention.
I also lost my freedom because I cannot make a trip or go anywhere without Effexor in my purse.”
Update January, 6, 2011
I forgot to post about some violent behavior I had at that time. I wrote about my experiences at the first year I was blogging.
I don’t feel like writing about it any longer. But I will do it if it helps people.
But those who come to this blog already know. So, it is almost useless. I gave up trying to raise awareness.
I’m trying to catch attention of those who profit from all of this.
Love to all of us!
The Human Cost of a Misleading Drug-Safety Study
A reexamination of old data for Paxil found that the antidepressant is more dangerous than the authors let on. How much harm has been done in the 14 years since it was published?
By David Dobbs
https://www.theatlantic.com/health/archive/2015/09/paxil-safety-bmj-depression-suicide/406105/
for it’s only rarely that researchers are able to crack open the tightly sealed file cabinets of drugmakers and look at raw trial data. This illustrates why they want to do so: It appears to be a direct demonstration of how a company and researchers can misinterpret the data to make a bad drug look good.
CONFIDENTIAL
Seroxat Article 31 – Consolidated Response Document – January 04
APPENDIX 6.
1.1. Narratives for Cases of Suicides in Clinical Trials
Table 1.1 Patient Identifiers of Patients who Completed Suicide during Paroxetine Clinical Trials
Table 1.2
Study Treatment Start and Stop Dates and Date of Death for Patients who Committed Suicide during Paroxetine Clinical Trials
https://www.gsk.com/media/1648/2004_appendix-06.pdf
On day 8 the patient committed suicide by hanging (preferred term: emotional lability). The investigator considered this to be unrelated to study treatment.
On 20 Jul 1984 the patient committed suicide by hanging. He had made no previous suicide attempts. The investigator considered the suicide was not directly related to study medication
She had made a previous suicide attempt shortly before her admission to hospital. The patient returned home from hospital on 22 Nov 1985. She committed suicide on 10 Jan 1986 by hanging herself. She had taken paroxetine30mg/day for over 4 months.
Patient 245.161.0163
Study Medication: Paroxetine Cause of Death: Suicide (jumped from window) The patient was a 67 year old female with no previous episodes of depression. She had had her current episode for approximately 3 months on entry into the study. The patient was started on paroxetine. She was not taking any concomitant medication.
On day 2, the patient committed suicide by throwing herself out of a third floor window. The investigator considered the suicide to be unrelated to study medication
Etc. Etc. Etc. …
Sad anniversary today: It’s been 5 years since my wonderful and courageous mum “passed away” after being poisoned by her GP and French medical system. Her GP had ignored my concerns about the side effects I had been noticing on my mum [even remotely as she was in France and I was in the UK] following his decision to prescribe a cocktail of AD a year and a half earlier because she was going through divorce and had lost her job.
When I went to confront him in person he told me ” We all prescribe AD, it’s not just me ” and threw me out of his office.
It wasn’t my mum who left that day – I remember how she started saying soon after the prescription of AD that “her brain was hurting”. That is not something she had ever said before. She had (very rarely) in the past said that she had a headache. I can’t imagine the pain of your brain hurting…but I’ve been living with the consequence for the past 5 years.
My mum was staying at my brother’s house. She disappeared on the 21/09/2016 leaving a note saying that she loved us: her children, her grand-children, her daughter-in-law, her son-in-law, but that she couldn’t take it anymore, she wrote: LES MEDICAMENTS !!!
We looked for her for 2 weeks, the local gendarmerie was useless.
It is my brother who found her body, in the forest just a few meters behind his house, she’d been there for 2 weeks, her feet above the ground.
I’ve contacted lawyers in France and made an official complaint but the case was closed before it even started. I’m nobody and they are too powerful.
I’m the proud daughter of Claudine Noelle Boudet who did not choose to die 5 years ago.
Nathalie, your report here is very powerful and illustrates so clearly the way that many of us have been dismissed by those who think they know better than us. Think they know better but behave in such a detrimental fashion. You are, indeed, the proud daughter whose pride will outshine the very worst that the “professionals” threw at you and at your mum – and also at the rest of your family of course. Our thoughts are with you.
Nathalie, thank you for this heartbreaking, yet powerful and deeply moving tribute to your mother Claudine Noelle Boudet. I am so very sorry for you, and for your family.
Inspirational and compelling lecture. Thank you. This should be a vital introductory address for all newly enrolled medical students. It would be invaluable for all graduate doctors commencing Vocational Training for General Practice, and for those commencing General Professional Training. Insight into, and awareness of pharmaceutical marketing masquerading as ‘Evidence Based Medicine’ would lead to safer and more thoughtful prescribing; thus improving patient safety.
Brilliant and educational. Thank you.
Your article reads like you have done some reading in philosophy of science, which is a good thing.
Turning your argument to look at a corollary, RCTs can be used to poison the well against competing, inexpensive, repurposed drugs.
There are special Russian word s which are applicable to the whole disgusting shenanigans ….. “maskirovka”… deception to mask the truth …and “vranyo” where the listener knows the speaker is lying, and the speaker knows the listener knows he is lying, but keeps lying anyway.