Rosie Meysenburg's story For anyone interested in the effects of drugs, the website SSRI stories has been an inspiration. Rosie Meysenburg, its creator, was recently diagnosed with cancer and is terminally ill. The story of how she came to create SSRI stories shows what people can do to hold the powers that be to account. —David Healy DH: How did you get started … [Read more...] about The Story of SSRI Stories
Archives for February 2012
Zoloft Study: Mystery in Leeds
In my blog post The best bias that money can buy I outlined how doing trials of their drugs in conditions like depression is the ultimate way companies hide bodies. That what is needed instead are studies of drugs in healthy volunteers. Here’s a good example of what a healthy volunteer (phase 1) study can show, and how the story of antidepressants and suicide might have … [Read more...] about Zoloft Study: Mystery in Leeds
The Best Bias That Money Can Buy
Randomized controlled trials (RCTs) were adopted by FDA in 1962 following the thalidomide disaster. This was a way to manage the risks posed by potential poisons. If the toxicity from a drug could be shown to overcome to some extent the toxicity stemming from the illness, a risk-benefit ratio would be set up that would warrant taking the risk of giving the poison. But what … [Read more...] about The Best Bias That Money Can Buy
Press Release: Pharmageddon is here
For immediate release Toronto, February 28, 2012. Pharmaceutical companies have hijacked healthcare in America, and the results are life-threatening.In his new book, Pharmageddon, Dr. David Healy documents a riveting and terrifying story that affects us all. Healy also has an idea for the solution..."A medical classic the day it was published." "Pharmageddon is a must-read … [Read more...] about Press Release: Pharmageddon is here
The Spin That No Data Can Overcome
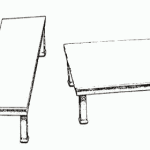
Roger Shepard's above illustration shows two tables of exactly the same size and shape. It’s an extraordinary example of how even when you know that the table tops are the same, the data changes nothing. The dynamics of perspective mean we continue to see things in the wrong way. Early on in the Prozac and Suicide controversy, Eli Lilly adopted a strategy that has “put … [Read more...] about The Spin That No Data Can Overcome
False Friends
‘Evidence’ is what the French call a false friend. You think you understand the word but you don’t. In French or Dutch, the Evidence in Evidence Based Medicine means that something is self-evident – as in using a parachute when jumping from a plane or penicillin for septicemia or an antipsychotic to tranquilize. You don’t need a clinical trial to work out what the right thing … [Read more...] about False Friends
Petra’s Story: Cymbalta
This piece is the first of a series showing people struggling with the Kafkka-esque absurdities of modern healthcare. It is written anonymously. If you'd like to share your story, please contact us. — David Healy A little over two years ago my daughter’s partner was killed in a tragic accident while in the company of my son. Naturally, this caused terrible grief and sadness … [Read more...] about Petra’s Story: Cymbalta
Randomized God
Several controlled clinical trials have recently been reported in which patients with cardiac conditions who were prayed for appeared to do better than those not prayed for (1, 2, 3). The surprise that prayer seems to do something has to be matched by surprise at the fact that its effects are relatively weak. If we are to build on this, we need to work out are these weak … [Read more...] about Randomized God
We Need Data for Data Based Medicine
One of the purposes of this blog is to invite colleagues to add to the knowledge base on drug groups. To submit a paper or to provide your comments, please contact us. I'll start the ball rolling with the following draft Data Based Medicine (DBM) papers: Antidepressants for Takers Antidepressants for Prescribers We have draft papers in preparation on: Mood … [Read more...] about We Need Data for Data Based Medicine
Coincidence a Fine Thing
Coincidence can be a fine thing. No sooner had I finished The tricks that drug companies do live after them, asking for examples of maneuvers to add to a generally available repository of tricks, than up pops Robert Gibbons' paper, Suicidal Thoughts and Behavior With Antidepressant Treatment, with not one but two maneuvers and reminders of others. Dangerous liaisons First … [Read more...] about Coincidence a Fine Thing