A year ago, Peter Selley, featured below got interested in the trials for an RSV vaccine. I paid no heed but Peter had smelt a story. Soon after I smelt a related story about efforts to enrol pregnant women in clinical trials.
Sixty years after we introduced Randomized Controlled Trials (RCTs) into the regulations governing the licensing of drugs, aimed at forestalling catastrophes like the birth defects caused by thalidomide, we were now for the first time running trials in pregnant women. A story, an arc of history, has come full circle. What could we, might we, learn from this.
There are two versions of this talk. One by me alone which is Here in English – it was due to be given in Bruxelles but was cancelled at the last minute because of venue issues. It has been translated into French by Ariane Denoyel – Femmes Enceintes et Essais Cliniques. The slides below feature in the Solo Video Version Here and in French version.
The other version involves both Peter and I and his set of slides which form the beating heart of this talk have some overlaps with and some differences from mine. Peter and David Duet is Here.
Slide 1: Peter Selley, whom you see above, is primarily responsible for the research on the RSV vaccines in this talk. I provide the framework which you might call the ethical or the marketing issues – its increasingly difficult to distinguish ethics from marketing.
Slide 2: My entry to these issues came when asked to write a chapter for a book being edited by bio-ethicists I know and respect. I assumed the book would feature the hazards of pregnancy research.
Not so. The book was in favour of more research on pregnant women and the chapter by Dee Mangin and I saying letting pharmaceutical companies near this area was a bad idea and that RCTs do not offer the gold standard evidence that people claim, was the exception.
The view the book was leaning toward seemed naïve to me. But strange things have happened lately. I’ve worked with lawyers who fought pharmaceutical companies on SSRI and other drugs and know all the tricks but who in the case of the Covid vaccines pretty universally figured companies like Pfizer will have played things straight. I found it hard to believe these hard bitten hombres thought this this way. I was more inclined to think Leopards don’t change their spots.
Slide 3: Here is – for me – the key slide in this talk. Its the Oval Officer and John F Kennedy has just signed the 1962 Food and Drugs Act and is handing the pen over to Frances Kelsey who stood in the way of the licensing of Thalidomide in the USA. She is the only woman in the room.
The 1962 Act was all about Drugs in Pregnancy. It replaced a 1938 Act which centred on Safety and the judgement of a woman like Kelsey with an Act that stressed Efficacy and replaced human judgement with an Algorithm. Regulation generally is about Safety – whether Food, Traffic, or Stock Markets. Its strange that the one place where Efficacy triumphs over Safety is in the case of Medicines – which are unavoidably risky.
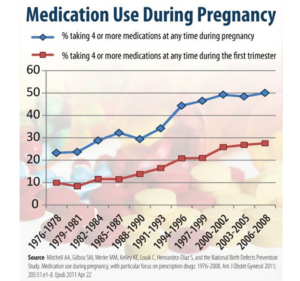
Slide 4: The 1962 Act may explain this intriguing graph which shows that at a time when women don’t take alcohol or even eat cottage cheese while pregnant, there has been a steady increase in the number of meds they take – white university educated women leading the way.
Slide 5: The US government is now pushing to get pregnant women in trials as part of an empowerment agenda. The chapters here all advocate the involvement of pregnant women in research, especially RCTs. Women, they say, are not vulnerable subjects. When pregnant, more than at any other time, they need to have good evidence and it is not good enough to find that doctors can offer none. These are all reasonable positions and they point toward Randomized Controlled Trials (RCTs) which it is believed offer Gold Standard evidence.
Slide 6: The changing climate is reflected in the imagery here.
Slide 7: When it comes to RSV research, Bill Gates has been a major donor both to studies in pregnancy and to efforts to change the climate about vaccines in pregnancy.
Slide 8: RSV vaccines have a history. In the 1960s, the first vaccine had a grim outcome – more children on the vaccine developed severe disease and died and we recognized the phenomenon of Vaccine Associated Enhancement of Respiratory Disease (VAERD) which has haunted respiratory vaccines ever since.
Slide 9: This article from 25 years ago make the case that any of us entering clinical trials hoping to benefit family, friends countrymen are in fact putting everyone we know in a state of legal jeopardy. How so? Well companies have decided that unless an RCT shows something to be happening to a statistically significant extent – then its not happening. So you get injured in a company trial and you vanish and companies use the absence of any injuries in their trials as evidence against anyone else who is then injured in trials on their drugs or vaccines.
The man you see here is Ian Hudson – the Chief Safety Officer of GSK – essentially stating under oath that our drug paroxetine causes no problems at all. This is at a trial triggered by Don Schell being put on Paxil and 48 hours later shooting his wife, his daughter and 9 month old grand-daughter and then himself. Hudson is saying there is no evidence our drug could have done this – the jury disagree and in fact GSK’s trials show their drug could do it. But Hudson later became the Head of Britain’s drugs regulator and this is the view ensconced at the top of all regulators – FDA – EMA and WHO.
Ian now works for the Gates Foundation and so his views continue to have some weight in the places that count.
Slide 10: This much more recent article covers Covid Vaccine consent forms, which seduce us into trials telling us that if injured call the investigator – giving the phone number, and that Pfizer and other companies have insurance policies – giving the number. But the companies have no intention of honouring this. People injured in the trial like Augusto Roux and Bri Dressen disappear.
The man in the middle is Mark Barnes, receiving an award as litigator of the year. Barnes is perhaps the single most interesting person in these developments. In 2009 Pfizer were fined over $2 Billion for failures to properly inform doctors of drug hazards. Soon afterwards Barnes who works for Ropes and Gray set up MRCT – which was AllTrials before AllTrials – set up after GSK were fined $ 3 Billion. MRCT and AllTrials completely support the Hudson view and MRCT have likely helped WHO issue instructions to clinical trialists in vaccine trials – to never link an adverse event to a vaccine in a trial. See Harmatology.
Slide 11: Clinical trials are dangerous for patients and terrible for doctors and the public given that we do not get to see the data – Not Even FDA sees the data. But if you are an investor in the company, a little over a decade ago, the US Supreme Court decided you have rights to see the data and make up your own mind as to what those data mean. You will see this is a pertinent issues in the RSV vaccine trials.
Slide 12: Finally here is part of the consent form for Pfizer’s RSV vaccine trial. You but not your baby are getting the vaccine and so there is no risk to your baby? Is this reasonable? And remember when getting recruited to a trial, a woman is likely to be lying on her back with some doctor having their hands on her abdomen.
You notice the word Advarra here. What we call Ethics Committees in Europe, were IRBs in the US but have now become businesses and consolidated down to 2 or 3 big companies – one of which is Advarra, in which Mark Barnes has a key role. These businesses often write much of a trial protocol, they then approve. They are among the most profitable businesses now to invest in – more so than the companies that run trials that can go wrong.
You are now going to hear a lot more details of other RSV trials. If you were a pregnant woman considering entering Pfizers’s phase 3 trial, should you have been told about what you are about to hear?
Slide 13: Which brings us to Pfizer’s RSV vaccine trial – Matisse – which is just led to licensing of this vaccine for RSV.
Slide 14: First we need to visit GSK. As part of a series of trials, they applied for NHS Ethics Committee approval for their RSV PED-003 vaccine. The committee were unimpressed with the idea of taking blood from infants just to measure antibodies – measuring infections was reasonable but antibodies are irrelevant and a lot blood tests on infants did not seem a good idea. GSK were given an unfavourable opinion and the trial never took place in the UK. It took place elsewhere but GSK has abandoned this vaccine.
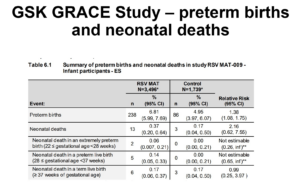
Slide 15: At the same time GSK were running their Grace study – an RSV PreF vaccine for pregnancy. This terminated early. Nobody except their investors were any the wiser as to what went wrong. No journal articles etc. The company later told regulators there was a premature birth issue.
Slide 16: It was a year before anyone else found out what was up. In a presentation, GSK gave figures for premature births but also neonatal deaths, which were doubled. They had no idea what was going wrong and no evidence their vaccine was the cause.
Slide 17: This slide at a recent talk gives some of the take home points – prematurity was commoner in women in LMIC countries. Having another vaccine, GSK said, lowered the prematurity rate but increased the difference between vaccinated and placebo – the vaccinated of course now had two vaccines and this happened while women were being encouraged to be vaccinated for Covid.
Slide 18: What do you make of this? This trial of a Covid vaccine in pregnant women was abandoned. You can believe the story that almost all pregnant women were already vaccinated if you want. Pfizer committed to keeping a pregnancy registry but this has never materialized.
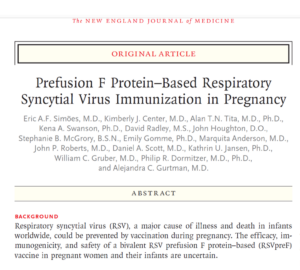
Slide 19: Against this background, NEJM publish Pfizer’s RSV vaccine Phase 2 trial. Usually an article like this looks like the final publication but now they are interim publications – and we may never have a final publication. This Weathervane publication claims the vaccine works well and is safe. The first author is Eric Simoes.
It is also important to note that Pfizer’s vaccine is almost identical to GSK’s – the one that caused premature births and neonatal deaths. The main difference lies in the adjuvants and other excipients.
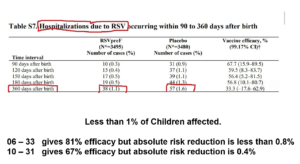
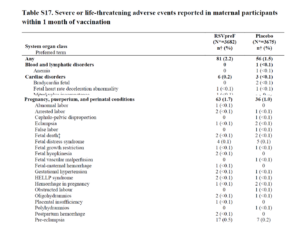
Slide 19: The paper states that there were 15 premature births across all groups in this trial – it does not tell us that 14 of these 15 were in the vaccinated group.
Slide 20: Peter noted there was an excess of cases of neonatal jaundice and wrote to NEJM about it – who took months and then refused to publish the letter.
Slide 21 Pfizer responded but not in a published letter. NEJM does not publish reports of adverse events. Their response says the vaccine didn’t directly cause jaundice but it avoids the elephant of prematurity in the room
Slide 22: Here is Eric Simoes – the lead name on the authorship line. Eric as it turns out was on the DSMB that stopped the GSK trial – on the basis of prematurity and neonatal death – so he knows that prematurity is an issue in these trials.
Slide 23: Here you note that Pfizer are also presenting to their investors before anyone else.
Slide 24: William Gruber, a senior Pfizer honcho and on the authorship line of the Phase 3 trial – is responding to an investment analyst saying yes GSK had a problem, but there doesn’t seem to be any problem with our vaccine.
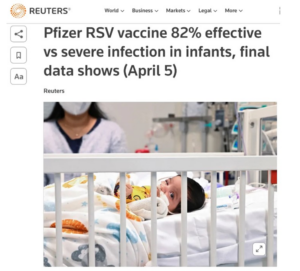
Slide 25: Here is the reading from the Pfizer Phase 3 Weathervane. It stopped early – apparently because the vaccine was so successful.
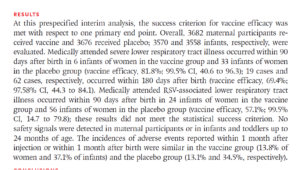
Slide 26: The results claim it was 82% effective and totally safe. The abstract tells us that the 82% comes from 6 RSV cases in 3682 vaccinated women against 33 cases in 3676 placebo women.
Slide 27: Here’s Beate the lead author saying the results were beyond excellent.
Slide 28: Here in Lisbon Beate is saying There was no statistically significant difference in the levels of prematurity between the two arms. This phrasing always means there was more prematurity on the vaccine or drug than on placebo. This thinking is key to company claims that RCTs never show any problems with our vaccines or drugs.
Overall the levels of prematurity were around 5%, Beate says, which is much lower than we would anticipate … which means that participation in clinical trials improves clinical care.
Slide 29: What Beate has not told you is that Pfizer have excluded anyone at any possible risk of a premature birth – Maternal Exclusion Criteria – Pfizer phase 3
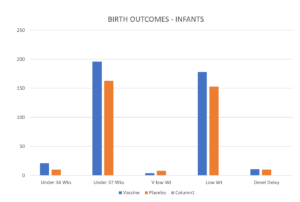
Slide 30: And Beate has not shown you this slide – a slide Beate has but did not show in her Lisbon presentation telling us all was fine with this vaccine. And at first glance it is not obvious why she wouldn’t show it. Over on the left are the vanishingly small number of very premature births.
But there are two tricks to this slide. One is the Y axis trick – If she had a Y axis going from 0 to 10 or 20 – the very premature figure would have looked much greater. The other trick is she is showing percentages.
Slide 31: So let’s change the Y axis – not to 10 or 20 but to 250 which should make Pfizer’s vax look even better and lets convert the percentages to figures. All of a sudden the prematurity issue looks more worrying. Its not just prematurity – its what is causing it.
Slide 32: Beate’s paper tells us that there was 82% efficacy based on 6 vaccinated infants getting RSV by 90 days postpartum compared with 33 unvaccinated infants from over 3600 mothers in each group.
But if you look at the data in the appendix, it says that the figures are 10 vaccinated and 31 unvaccinated and critically these are from less than 3500 women in each group. Taking these figures, vaccine efficacy is 67%.
The nearly 3600 figure refers to women screened but nearly 200 in each group did not enter the trial, It is not clear to me how Pfizer managed legitimately to extract an 82% figure from these data but they grabbed at it. Whatever the right figure, apparent efficacy seems to vanish pretty quickly after 90 days.
Whichever you opt for – 82% or 67% – this translates into less than 0.8% absolute risk reduction or roughly 0.4% absolute risk reduction.
You can see RSV in these trials was less than 1% anyway in the placebo group and vaccination reduces this risk by a fraction of 1%. Breast feeding is a better bet in terms of handling RSV.
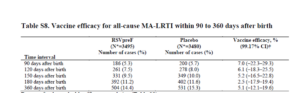
Slide 33: That was Table 7 in the appendix, this is Table 8, where you have what Pfizer claim are all medically attended lower respiratory tract infections – you can see overall that there were just the same number of respiratory infections in vaccinated and unvaccinated.
Slide 34: The consent form for the trial shows these women are being paid. Paid if they need to attend for symptoms of vaccine reactivity – confirmation of which breaks the blind and likely comes with a message – well at least your child won’t get RSV.
Paid if they bring their infants along with suspected RSV – which unblinded placebo patients may be more likely to do.
So how dependable are Beate’s figures?
Slides 35 This slide shows a higher rate of complications among the vaccinated mothers – 81 to 56 overall and 63 to 36 specifically for perinatal issues among which are pre-eclampsia a very serious condition 17 – 7.
Neo-natal jaundice is a consequence of prematurity but pre-eclampsia might be a cause.
Slide 36: The problems don’t stop there. This Danish study looking at delayed onset of RSV in the Covid pandemic because of lockdowns and masking suggests that if children don’t get RSV in the first year of life – they get a more severe episode if they first contract it later. This may be like polio – harmless in the first year of life, disastrous if caught later.
The effects of RSV vaccines don’t last – will this make RSV more dangerous if contracted later?
Slide 37: Putting all this together it is not unreasonable to wonder if Pfizer didn’t find a way to terminate this trial early because things were going badly wrong. Claims for 82% efficacy and complete safety look deeply misleading.
Slide 38: Here is an innocent looking paper from the Lancet that repeats stock ghostwritten phrases about 33 million cases of clinically significant RSV each year leading to hospitalizations and deaths. If you read closely you see these mostly come from low and middle income countries
Slide 39: Less innocent when you look at pages 2 and 3 which are taken up with one of the longest authorship lines you will ever see. For a treatment aimed at low and middle countries, oddly most of the listed authors are Westerners.
Slide 40: Same story again and again in the Lancet. I could show you endless articles like this – with an almost identical drumbeat.
Slide 41: And these all echo the messages on the ReSViNet Website, which is that 33 million children globally suffer from RSV, leading to thousands of deaths. For the sake of humanity we need to tackle this.
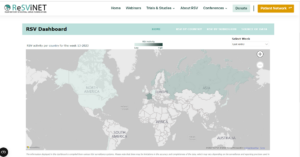
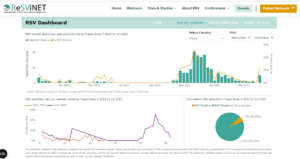
Slide 42 What ReSViNet has done is to recreate the dashboards that were such powerful motivators during the Covid epidemic – they generated a sense of crisis – that something needed to be done to turn the numbers around – just like numbers do when we step on weighing scales or get screened for bone densities.
Slide 43: In the slide above you have France picked out and here you have the data for France – illustrating that RSV has seasons and we risk losing children unless we protect them before the season starts.
This is the kind of thinking that creates Cults – when you are under pressure to convert now or risk eternal damnation.
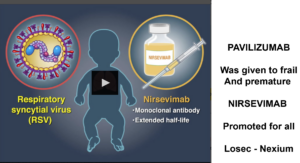
Slide 44: The ReSViNET Dashboard was sponsored by Astra-Zeneca. They don’t have a vaccine in the mix so what’s up? For over a decade there has been a monoclonal antibody – palivizumab – which has been given to some vulnerable children born around RSV season time. Giving Monoclonals to anyone is risky, to infants even more so but perhaps justifiable if used with care in children at risk.
As it came to the end of its patent life palivizumab was bought by Astra-Zeneca and is being retired and replaced by Nirsevimab which is more likely to be offered to all infants born near RSV season time.
Slide 45: In the UK, a few well placed Ladies in the House of Lords appear to have taken it on themselves to help generate a sense of crisis. They have intervened on several occasions to ask the government what is it going to do to avert the looming crisis. Remember this was supposed to be a disorder affecting low and middle income countries and the biggest thing that can make a difference is breast feeding.
Now I am going to use a Celtic prerogative here to ask – will these girls be taking their monoclonals and getting their vaccines.
Slide 46: Magic Johnson is playing front man for GSK on spreading the word about the approval of GSK’s RSV PreF vaccine for older adults n- over 60 that is. RSV is a killer for older adults and you all need to get it – just like the Shingrix vaccine.
Slide 47: Pfizer’s vaccine for older adults also looks likely to get approved although it is linked to Guillain-Barre syndrome.
Slide 48: We can look forward to some great GSK and Pfizer ads on the lines of Oh Granny .
Slide 49: There are a lot of similarities between the RSV and Covid stories.
- Close cooperation between Bill and Albert
- A ghostwritten literature
- No access to the trial data
- Publication in New England Journal
- Which means no need to worry about side effect reporting
- Dashboards and marketing by Fear
Slide 50: Here is Banquo’s ghost come back to the feast – I wonder if MacHudsons or MacGates are reacting. He is saying in this article that none of our vaccines for primarily respiratory conditions are any good. We still haven’t worked out how to do them right.
Slide 51: Let me take you back to Frances Kelsey and the Oval Office where Safety is being replaced by Efficacy.
Should women get involved in company trials? Will this empower them?
In 1962, Louis Lasagna was the person who transformed medicines regulation from safety to Effectiveness. Two years before that he had run a placebo-controlled trial of thalidomide demonstrating it was a good sleeping pill but this trial missed the agitation, suicidality, sexual dysfunction and peripheral neuropathy it caused.
RCTs are the gold standard way to hide adverse events. Worse again because they can give the appearances of efficacy by producing benefits on a surrogate outcome they generate pressure to take the treatment even though we have no safety data. Women participating in these trials are increasing the risks for all other women.
Slide 52. If you want to see more about why RCTs are the standard through which industry make Gold rather than something that offers women good evidence – see this post on DH.
Slide 53: So as part of our introduction to Does My Bias Look Big in This – we wrote that RCTs are not the best option for generating evidence for women pregnant or thinking of getting pregnant. The best evidence would come from pregnancy registries listing all treatments a woman has had in pregnancy and all outcomes.
These you will say aren’t randomized or controlled. This is true – assessing what they mean will be up to the individual women looking at them.
Registries are better than RCTs for two reasons.
- RCTs tell us about a possible effiicacy and this comes sold as an obligation to take it – just like the obligation to correct your serotonin levels. What we really need is some way to gauge safety and a registry is the best option for this.
- Women who are or who are thinking of being pregnant are likely the most judicioius and responsible people on the planet – I have confidence they will on balance make much better judgement calls than pharmaceutical companies – or anyone else or that matter.







































Looks like Pfizer DOES have a Pregnancy Registry for its Covid-19 vaccine! And while their clinical trial for pregnant women/newborns hasn’t shown any results yet, the Registry is publishing already. Needless to say, the news is good-good-good:
https://onlinelibrary.wiley.com/doi/10.1002/pds.5600
The Pregnancy Registry is in the hands of a specialized CRO called Pregistry.com (recently bought out by a larger CRO called Corevitas.com). It goes by the reassuring name of C-Viper (no, I did NOT make that up).
https://www.themiddlemarket.com/latest-news/audax-backed-corevitas-acquires-pregistry
https://c-viper.pregistry.com/
For its RCT, Pfizer relied on dozens of private for-profit clinical-trial mills including the notorious Ventavia in Texas. But hey, at least we know where the research is being done. With Pregistry in charge, we’ll know just about zero. As for the Pregnant People who join the Registry, looks like they will be participating remotely through their phones or handy tablets. No doctor or nurse whose physical door they can knock on if they have something urgent to report.
I agree that RCT’s are not enough – not by half – and Registries are urgently needed to monitor pregnancy outcomes in the real world. But with Pfizer in charge of both, how much good can we expect from Registries?
BMJ 2023; 381 doi: https://doi.org/10.1136/bmj.p1223 (Published 30 May 2023)
Cite this as: BMJ 2023;381:p1223
Jeffrey K Aronson
Author affiliations
A new report on the conduct of commercially sponsored clinical trials of new medications has just been published. It contains 27 recommendations. The UK government has welcomed all of them, and has promised to fund five. Important features of such trials that are not mentioned in the report include methods for improving and measuring participant adherence to an intervention and methods for detecting harms.
A review
Earlier this year, the UK government appointed Lord James O’Shaughnessy, senior partner at a consultancy firm, Newmarket Strategy, a board member of Health Data Research UK, and a former Conservative health minister, to conduct an independent review into what was described as “the UK commercial clinical trials landscape.”1
The background to this review was that there had reportedly been a 44% fall in recruitment of patients to phase 3 commercial clinical trials between 2017 and 2021. This had had the effect, according to an analysis by the Association of the British Pharmaceutical Industry (ABPI), of dropping the UK down the global rankings for numbers of phase 3 industry sponsored clinical trials from fourth place in 2017, after the USA, Germany, and Spain, to 10th in 2021, now behind the USA, China, Spain, Japan, Germany, Canada, France, Poland, and Italy.2 This was despite the fact that during the same time the UK had risen from fourth to third place in regard to phase 1 trials and from 10th to second in regard to phase 2 trials. The implication was that there were difficulties in recruiting patients to the phase 3, so-called pivotal, trials that companies use in applying to regulatory authorities for marketing authorisations.
However, rather than analysing the raw numbers of trials being carried out in each country at each phase, expressing the numbers of trials being carried out per million population would have given a better reflection of what had happened in different countries. China, for example, was second only to the USA in the ABPI’s 2021 table in terms of the total numbers of all three categories of trials, phases 1, 2, and 3; however, it was 10th in the table when the data were corrected for population, and would perhaps have been even lower, had other countries’ performances also been reported.
By a per million population analysis, the UK’s performance stayed steady at fourth place globally in regard to phase 1 trials, fell slightly—from fourth to fifth place—in regard to phase 2 trials, and fell from sixth to seventh place in regard to phase 3 trials. So the drop in performance on this measure compared with other countries was not as great as the ABPI claimed on the basis of the absolute numbers of trials being performed. Nor did the ABPI include information on the sizes of the trials performed, nor the innovativeness of the products being tested, nor the outcomes.
Nevertheless, as a crude measure, the numbers of trials per million population in the UK did fall markedly, by almost 50%. Despite that, the UK has more or less held its position globally, because fewer phase 3 trials have been carried out everywhere, falling from 2784 by the top 10 countries in 2017 to 2126 in 2021. In contrast, the numbers of phase 1 and phase 2 trials have not changed. This is consistent with the fact that most failures in drug development occur at phase 2,3 when the early promise of a new medication is not fulfilled, although failures can also occur at phase 3.
Other reasons may therefore at least partly explain the reduction in the numbers of phase 3 trials besides difficulty in recruitment. For example, in 2021 fewer drugs were newly licensed in the UK (35, of which few were regarded as innovative) than in the EU (40) or the USA (52).4 A more granular analysis is needed.
Lord O’Shaughnessy’s review has just been published,5 having been carried out with commendable speed, so quickly in fact that one might be forgiven for unworthily wondering whether at least some of the recommendations to which the government would be likely to agree were decided in advance. The report runs to 74 pages and includes 27 recommendations relating to eight stated problems. The government’s response has been published simultaneously, supporting the recommendations and detailing agreement to implement five of them, at a cost of about £120m over the next three years.6
A detailed analysis will require more time for reflection. Instead, I should like to highlight aspects of clinical trials that are not mentioned in the report.
Trial quality and participant adherence
Many factors contribute to the quality of a clinical trial. Absence or at least minimisation of biases, for example; a high rate of adherence to the protocol; participant adherence to the intervention; and a low rate of drop-outs. Of these, participant adherence is perhaps the most neglected.
As the former US surgeon general C Everett Koop once said, “Drugs don’t work in patients who don’t take them.” Poor adherence in clinical trials has several potential consequences, leading to biased results, reduced statistical power, and impaired causal inferences.7 These include:
● failure to confirm efficacy or underestimation of the extent of efficacy;
● by the same token, underestimation of the risks of harms;
● impaired development of innovative drugs and drugs for rare diseases.
Lord O’Shaughnessy’s report says nothing specific about how good participant adherence can be achieved and measured. Adherence to trial protocols gets a passing mention.
Harms
The word “harm” does not appear in the report in relation to the adverse effects of trial interventions. The term “side effects” is mentioned in relation to phase 1 studies. Harm from clinical negligence is mentioned. Terms such as “adverse events,” “adverse effects,” and “adverse reactions” do not appear. The word “risk” appears several times, but not in relation to adverse drug reactions or a comparison with benefits, the so-called benefit-risk ratio, better termed the benefit to harm balance. A single reference to “the risk assessment process” might be related to adverse drug reactions, but could equally apply to “the risk, or perceived risk, of carrying out clinical trial activities to the trial sites themselves,” mentioned elsewhere.
Most early phase clinical studies are too small to detect anything but the most frequent harms. And there is a risk that by rushing to approve a new medication that appears to be highly beneficial, important harms will be missed.
Take, for instance, lorcaserin. The history of medicines that have been developed in the hope of managing obesity has featured more withdrawals than survivals. When we surveyed medicines that had been licensed for use in the management of obesity since 1950, we found that 25 had been withdrawn from the market because of adverse drug reactions.8
Lorcaserin, a selective 5HT2C receptor agonist, developed for use as a weight reducing agent, gained marketing approval for obesity in the USA in 2012, albeit after initial hesitancy by the Food and Drug Administration (FDA) because of concerns about harms. Concerns about the risks of tumours, psychiatric disorders, and valvulopathies led the European Medicines Agency (EMA) to refuse the manufacturers of lorcaserin marketing approval. When we reviewed the evidence,9 having analysed the results of a large clinical trial in overweight or obese subjects with high cardiovascular risk profiles, whose authors concluded that lorcaserin generated sustained weight loss without increased risks of major cardiovascular events, we came to a different conclusion. We concluded that lorcaserin caused minimal weight loss, that its harms profile was as yet incompletely understood, and that it was unlikely to be a cost effective intervention. We also noted the long list of exclusion criteria for participation in the trial and thought that the published results were not likely to have strong external validity. A few months later, the manufacturer withdrew lorcaserin from the market, at the request of the FDA, because of adverse effects.10
A final thought
Innovation in pharmacological interventions is desirable, as is rapid development of new compounds to improve public access to effective medications. However, any rush to develop new pharmacological interventions should be tempered by assuring that phase 3 clinical trials are of high quality, by paying attention to such matters as participant adherence and the potential harms of newly developed medications as well as their benefits, in an attempt to maximise the benefit to harm balance.
Footnotes
Competing interests: JKA has written widely about adverse drug reactions, including publications in peer reviewed journals, edited textbooks, and medicolegal reports, mostly to coroners. He is a member of a consortium interested in the problem of adherence to medications and other therapeutic interventions.
There are so many scandals involving the callous way preganant women and their babies are mis -treated in UK hospitals that it is no surprise they are about to be abused again by the use of fear mongering and bullying when at their most sensitive and vulnerable. Very will have access to the concerns being flagged up in such as the Telegraph ( I don’t subscribe to it myself)
Ministers’ had ‘chilling’ secret unit to curb lockdown dissent (Hardly a ‘Secret ‘ )
The Telegraph
https://www.telegraph.co.uk › news › 2023/06/02 › co…
2 days ago — Prof Carl Heneghan, Molly Kingsley and Dr Alexandre de Figueiredo were monitored by government disinformation units
Ministry of Truth — Big Brother Watch
Secretive Whitehall units have been monitoring government critics’ speech online – including MPs, academics, journalists, human rights campaigners and the…
.28 Jan 2023
Juan Gérvas Retweeted
Molly Kingsley
@lensiseethrough
Following the @Telegraph news story today on censorship, the attached photos are the list of my articles, tweets and comments flagged by the Counter Disinformation Unit as potential ‘disinformation’.
It starts in 2020, continues throughout 2021 and into 2022 and even 2023, and covers topics that were ostensibly controversial (eg, questioning the vaccine roll-out to kids) and those that should have been incontrovertible (eg, “let children use playgrounds, MPs demand”).
We don’t know definitively how much of this translated to censorship, but like many critics of the Government’s pandemic ‘truths’ — lockdowns, school closures, masking children, vaccinating healthy children — I had a recurring sense that my social media accounts were being hobbled or suppressed in some way.
The @UsforThemUK social media accounts received repeated warnings that the group would drop down in terms of priority on newsfeeds if we didn’t remove content flagged by Meta as ‘disinformation’. For many individuals, accounts were expressly suspended or shut down.
Circumstantially, then, it’s not unreasonable to wonder whether monitoring at times traversed into censorship.
I feel vindicated in my suspicions if also saddened by the arrogant authoritarian overreach. But the most serious implication of all this is that for an extended period, one narrative has been amplified and another subdued. Many of the positions that I and others targeted by the CDU had advocated would, I believe, not only have been viewed as reasonable positions to take pre-2020, but to argue against them would have been considered extreme.
If you doubt that is true then consider how society would have judged anyone in 2019 who advocated mandatory isolation of children for weeks at a time; masking of children for 8 hours a day including through lessons to ‘mitigate’ against a virus which never seriously threatened them; injection of children with novel products despite no long term safety data existing. None of these were moderate policy decisions. They only became legitimised and normalised because dissenting voices were systematically suppressed.
Given that the information now being flushed into the public domain stems from only a small number of individuals who have submitted subject access requests, we should assume that today’s examples, and those previously detailed in the @BigBrotherWatch report, are the tip of a larger iceberg. Adding to the work of the Government’s spy units were fact checkers and ‘trusted news’ initiatives which further skewed discourse, and of course the outright shaming and smearing of those of us who argued against what we saw as the descent into a dangerously illiberal and unethical regime.
The reality of 2020-22 is that we were living through a period of State-sponsored extremism to which virtually no challenge in the public square was permitted. It hardly needs pointing out that this is at extreme odds with the notion of liberal democracy with which we tend to associate much of the developed West. All of this could be examined by the Covid Inquiry, but of course today’s revelations also make it less likely that a meaningful, successful, public inquiry is now possible.
https://twitter.com/lensiseethrough/status/1665061479761027075
Prof Norman Fenton
@profnfenton
·
11h
As I doubt the UK Govt “Counter Disinformation Unit” will counter the disinformation from its own @BBCNews how about they save the taxpayers £millions and close it down? Same goes for the 77th Brigade spying on UK citizens. Thanks @Johnincarlisle
UK government, Counter Disinformation Unit (‘CDU’)
https://www.youtube.com/watch?v=yxKL_BPVl3E
“This should work both ways, perhaps”…
Close Shave Down Under
Your antipodean cousins are gearing up for winter with their first ever RSV Awareness Week,
spearheaded by breakfast TV presenter Karl Stefanovic.
https://youtu.be/xDiwRCPkeR0