A paper looking at antidepressants and birth defects in Denmark has just appeared. Anyone can download it and read for themselves (Jimenez-Solem et al 2012). It’s worth reading.
The published data demonstrate an increased rate of major birth defects on SSRIs which fits what almost all other studies have found. But this study also finds that women who have stopped their SSRI 6-9 months previously are at a similarly increased risk.
This has led some of the authors apparently to say that the problem may stem from the underlying depression rather than its treatment. The paper puts it in a different way – there is something about the redemption of a prescription for an SSRI that leads to birth defects.
This is an extraordinarily worrying paper – perhaps one of the scariest in recent years.
The authors make the usual point about more research being needed. No paper is perfect and in this case there are known hazards with the method (prescription redemption records) the authors have used. Prescription redemption records fail to pick up many of the people taking a drug – and this might therefore explain why in this study relatively few Danish women register as being on antidepressants.
What do the data show?
First the children born to women who are on or have been on an SSRI have a roughly doubled rate of heart defects even though many major heart defects will be terminated. The higher the dose of SSRI the woman is on, the higher the risk of a heart defect in her baby.
The risk of a neural tube defect is no higher – but almost all neural tube defects are terminated in Denmark. The rates of termination in other countries looked at are higher in women on SSRIs, so this is presumably also the case in Denmark.
In contrast, the children born to women on other antidepressants do not have this increased risk. This is a particularly interesting finding in the Danish study in that in the 1980s the Danish University Antidepressant Group (DUAG) ran studies demonstrating that tricyclic antidepressants (TCAs) work in severely depressed patients when SSRIs are ineffective. So it seems a reasonable assumption that the women in the TCA group in this study were more likely to be severely depressed than the women in the SSRI treated group but the women on TCAs do not have as high a risk of birth defects.
There is some risk of birth defects in the TCA group – not as high as in the SSRI group – but this most probably reflects the fact that some TCAs, like clomipramine and imipramine, are also serotonin reuptake inhibitors and some like desipramine and lofepramine are not. In the same way some antihistamines like diphenhydramine and chlorpheniramine inhibit serotonin reuptake and some don’t. Those that inhibit serotonin reuptake cause birth defects. Those that don’t inhibit serotonin reuptake don’t cause birth defects.
Crucially with antidepressants other than TCAs and SSRIs do not have an elevated risk of birth defects.
What’s going on?
So what’s going on in the women who have stopped antidepressants for a few months but still seem to be at high risk of birth defects?
One option was outlined in Herding Women. Maybe these women, worried about the effects of taking something that sounds as unnatural as an antidepressant, figured that they’d switch to something more natural like St John’s wort, unaware that this also comes with a high risk of birth defects and miscarriages. Or maybe they switched to an antihistamine.
But the much more worrying option is this. SSRIs have far more enduring effects on the reproductive system and its related endocrinology than they have on mood. They shrink ovaries and testes and it is this that gives rise to the loss of libido associated with their use, which can sometimes be permanent. They reduce sperm quantity and function. These drugs really do have the kind of effects on testes and sperm that masturbation was once thought to have. Masturbation never did this, SSRIs do.
Before and after SSRIs
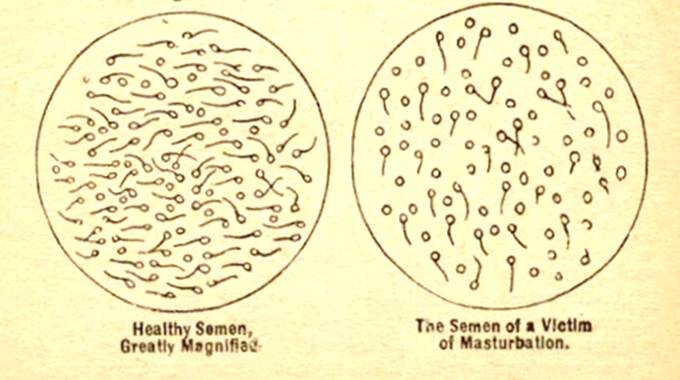
Ironically there is a striking similarity between this image and the usual image in advertisements of what SSRIs supposedly do – except of course the direction is reversed. There is no more evidence that this is the true effect of SSRIs on brain serotonin than there ever was that masturbation had the effects on semen outlined in the slide above.
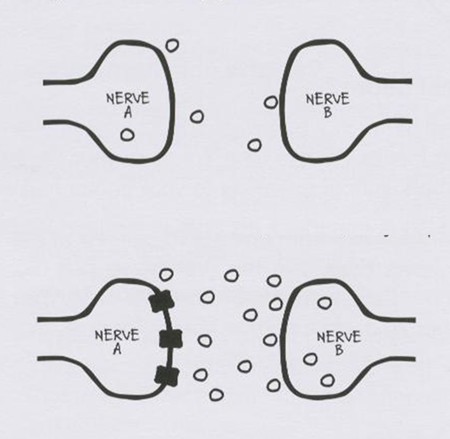
Given comparably powerful effects throughout the endocrine system, effects that are far more substantial and enduring than any effects that the kinds of anxiety or depression for which SSRIs are given have on the endocrine system, it is much more likely that it is some direct effect of the SSRI that is the source of these birth defects in women than leaving their anxiety untreated has caused the problem.
Before the SSRIs came on stream, women who were diagnosed as depressed had a far more serious condition than women treated with SSRIs have now. This condition was variously called melancholia or endogenous depression. This is a condition that does lead to endocrine disturbances – raised cortisol levels – but melancholia has not been linked to birth defects.
Back to the Middle Ages
The hypothesis that there might be a link between the kind of anxiety or milder mood disorders for which women get SSRIs now – in which there is no abnormality of cortisol – to birth defects is close to unprovable. What woman isn’t anxious at some point during her pregnancy?
Creating a culture where women were told that being anxious might cause their unborn child to have a birth defect sounds suspiciously like taking us back to the Middle Ages when women were told that birth marks on the faces of their children were caused by the mother looking at a fire.
It is far more likely that the risk of birth defects in women who have stopped their SSRI is mediated through some enduring epigenetic change or effect on the endocrine system brought about by previous treatment than it is that these birth defects are caused by untreated nerves. If this is the case, it means we do not know how long women have to stop SSRIs and other serotonin reuptake inhibiting antidepressants before it is safe for them to conceive.
Few doctors inform women of child-bearing years of the risks of dependence on an SSRI. Few inform women of the risks of birth defects on an SSRI. What is the right thing to say in the light of the most recent evidence? What would the American Woman who haunts these posts have to say?
There are several disquieting issues contained in this study, apart from the obvious incidence of birth defects. The authors state: “We divided the study population into pregnancies exposed to high or low SSRI dose based on the recommended daily dose values of the individual SSRIs during pregnancy. Doses over the following cut-off values were considered as high doses: 20 mg for citalopram, 10 mg for escitalopram, 20 mg for fluoxetine, 20 mg for paroxetine and 50 mg for sertraline.” They failed to consider the relevant half-lives of these drugs. For example, the half-life of fluoxetine and its active metabolite norfluoxetine is respectively 2 to 4 days and 7 to 15 days, much longer than other SSRIs. Most drugs are not intrinsically toxic to the liver but can
cause injury secondary to the production of a liver-damaging drug metabolite. In terms of medications, a metabolite usually refers to the product that remains after the drug is broken down (metabolized) by the body. We really don’t know how long this liver damage can last but if we infer that it is related to the half-life of the drug itself, as well as to the particular area of the liver affected, it could be many weeks or even permanent. It might also account for the somewhat lower, but not absent, risk of birth defects in the TCA group.
Also missing is an indication of what other medications they might have been taking. Drug-drug interactions require close scrutiny.
They also, as is common, did not take into consideration the influence of the
method of diagnosing depression although one might guess that it was often the self-report of something like the Beck Inventory. We appear to have lost the ability not only to identify but even to remember that melancholia is very different from dysphoria, dysthymia and anxiety alone. As has been pointed out, some degree of anxiety occurs in pregnancy, especially first ones. From the data, it appears that with each successive pregnancy, exposure to SSRIs decreased but so did pausing during pregnancy. These last may have been women who had not had children with birth defects and who, therefore, saw no potential harm but this must remain speculative.
And, there’s something about Harry, too.
James F. Paulson, Sharnail D. Bazemore, Prenatal and Postpartum Depression in Fathers and Its Association With Maternal Depression: A Meta-analysis.
Prenatal and postpartum depression was evident in about 10% of men
in the reviewed studies and was relatively higher in the 3- to 6-month postpartum. period. Paternal depression also showed a moderate positive correlation with maternal depression. JAMA. 2010;303(19):1961-1969.
Changing nappies can be very mood altering.
I find the article mentioned in this blog very interesting, and somewhat groundbreaking. Regarding the halv-lives of SSRIs, I believe they were taken into considarationby analysing stop of treatment 3, 6, and 9 months before pregnancy. Fluoxetines metabolites could in theory (depending on other pharmacokinetic factors) up to 75 days, which is less than 3 months. Do you have any information on how a damaged liver can cause offspring with congenital malformations?
Thanks for information on the JAMA article
I don’t think this has anything to do with half life – I suspect its much more likely to be the enduring endocrine changes these drugs cause than anything to do with half life
You might be right. Allthough it will be very diffcult to prove. another issue which is important, is that women taking antidepressant medication during pregnancy, as a group, have a much more unhealthy lifestyle than the background population. They smoke more, weigh more, eat less helthy food, excercise less and so on. These factors are also known to be very important in the development of the fetus, and are definetly linked to congenital malformations. I believe we are too focused on finding one cause to every symptom but it’s probably a combination of many factors. If a drug has dangerous side effects it has to be illuminated but we must not forget the remaining factor and risk condemning a drug thtat could improve lives for a lot of people.
The biggest obstacle to raising appropriate alarms about the effects of SSRI’s in pregnancy is the widely-promoted notion that depression itself can harm the developing child. This is repeated everywhere, and used to persuade both pregnant women and their doctors to keep those meds coming. I just found this write-up of a recent Dutch study which is said to prove that “both depression and SSRI’s pose risks.” The write-up appears to have a heavy element of spin:
http://www.womensmentalhealth.org/posts/measuring-the-effects-of-antidepressants-versus-untreated-depression-on-pregnancy-outcomes/
“Infants born to mothers with prenatal depressive symptoms showed reduced body growth and head growth. The risk of preterm birth in the depressed group (6.3%) was similar to that observed in controls (5.1%). Prenatal SSRI exposure was not associated with reduced body growth but was associated with reduced fetal head growth. The SSRI-exposed children were at higher risk for preterm birth (odds ratio = 2.14) compared to controls.”
“From this data, the researchers concluded that both depression and SSRI exposure may negatively affect outcomes. They noted, however, that the effects may be different. While SSRI exposure increased the risk of preterm birth, exposure to depression did not. Exposure to depression had a negative effect on overall growth; SSRI exposure affected only head growth.”
Whoever wrote this summary studiously avoided giving a pre-term percentage for the SSRI-exposed children, but as I read this, it must be about 11% — i.e., not only higher than the controls, but higher than the children of “untreated depressed” mothers. They tried to put a similar positive spin on the “growth” issues. I am not a doctor, but I would rather take my chances with a child who was somewhat undersized in general than one who was ONLY SMALL IN THE HEAD. Am I right?
The other major problem is that the “depressed but unmedicated” mothers were admittedly more likely to be poor and had higher rates of smoking, drinking and cannabis use. I don’t know how things stand in the Netherlands, but in the USA they would also be less likely to be getting decent prenatal care or adequate nutrition. Have there been any studies – say, some of those cited by Dr. Urato in his post last week – that took apart the influence of factors like these vs. the influence of depression itself on the fetus?
Here’s the actual article, which would be worth a closer look by folks who know what they are doing:
El Marroun H, Jaddoe VW, Hudziak JJ, et al. Maternal Use of Selective Serotonin Reuptake Inhibitors, Fetal Growth, and Risk of Adverse Birth Outcomes. Arch Gen Psychiatry 2012; 69(7): 715-721
http://archpsyc.jamanetwork.com/article.aspx?articleid=1151023
I agree, there’s always the argument that maternal depression is a serious health risk, with no mention of the definition of “depression” (which most likely is the rare, more severe type with lower life expectancy because of suicide); where that information comes from (may be only one study); and that non-drug treatments can be quite effective, not affect the fetus, and give the mother coping skills that will serve her well for a lifetime.
Instead, the proposal is to endanger the fetus (birth defects are forever) rather than more carefully prescribe antidepressants. It’s too hard for doctors!
“Before the SSRIs came on stream, women who were diagnosed as depressed had a far more serious condition than women treated with SSRIs have now.”
I didn’t understand why depression after SSRIs were more serious.
Al I know is that after SSRIs women and men change one antidepressant to another till there is no label left to try.
Sometimes ECT is prescribed.
Labeling Melancolia or whatever makes no difference: before and after SSRIs the only change was that “side effects” were added to the suffering of women who are depressed.
They suffer from the disease, the side effects, the withdrawal symptoms while changing one to another…
The most sad is that some are so lost that they don’t recognize what is depression, side effects and withdrawal.
Some end up their suffering by committing suicide.
But was it a suicide because she decided to end the suffering, because of the depression or a drug-induced suicide?
Ana – a substantial number of those who were endogenously depressed before 1990 had a severe condition that came with high risks of suicide, and raised cortisol levels. We in fact had a great diagnostic test to pick out those (few) who had hormonal changes and would respond to the older more potent antidepressants. Since 1990, anyone who is unhappy is labeled as being depressed and given SSRIs which are ineffective for “proper” depression
This study brings alarming news and should be discussed in the national press to help warn young mums. If it was a study showing that yet another supposed gene had been found for so-called schizophrenia – we would all have known about it.
In response to your comment David: Bearing in mind that high cortisol is associated with depression e.g cushing’s syndrome, might not this so-called endogenous (from within) depression actually be exogenous (from outside) as a response to chronic distress, stress or trauma through raised cortisol levels (HPA type response) that have the effect of, over time, damaging the negative feedback regulation that would normally allow the cortisol levels to fall/fluctuate? I am, perhaps naively, questioning the neat diagnostic entity of endogenous depression which I think you will probably want to defend! 🙂
Dr. Healy,
You wrote in reply to Ana “We in fact had a great diagnostic test to pick out those (few) who had hormonal changes and would respond to the older more potent antidepressants.”
What is this test? Why don’t doctors administer this test to those patients who exhibit severe, treatment-resistant, life-long depression?!
My doctors (general practitioner, psychiatrist and therapist) are recommending “mood stabilizers.” I’m resisting based on fear of side effects. They say we have to look at risk vs benefits. Are the potential side effects of these older medications similar to those of antipsychotics?
I tried all the different SSRIs. I’ve been on sertraline for more than 15 years. My dose for the past several years is 200mg/day. I’ve tried adding Effexor and Wellbutrin (separately, of course) to the sertraline. Neither helped.
Why can’t the psychiatric industry come to some sort of consensus about all this depression and medication stuff?! It’s left to scared, sick patients to try to figure it all out on their own.
I’m a 37-year-old, single female with no children (I decided several years ago never to have children, in part, based on the effects of anti-depressants.). I lost my job 5 years ago because of depression and I’ve been on disability for two years. I’ve essentially given up hope that I’ll ever know a life without near constant depression.
~ rl
The test was the Dexamethasone Suppression Test, based on the work of Barney Carroll, which was at least as good in picking up treatment responsive severe depression as EEGs are for detecting true convulsive disorders. The story of the evolution of this test and the politics that led psychiatry to abandon it are told in Endocrine Psychiatry by Shorter and Fink.
I seem to recall that the older antidepressants, particularly the MAOIs, were superbly effective in endogenous depression with a high risk of suicide yet today it is impossible to get a psychiatrist even to consider their use. I currently have a friend whose husband is, in my opinion, a high suicide risk and a perfect candidate for phenelzine yet when suggested, the reaction is a though one had proposed cupping and bleeding combined with spells and incantations under a full moon. I’ve reached a point at which I’m seriously considering sticking pins into small wax dolls representing those who will not listen, or who listen but ignore.
This unfortunate man is now being changed from fluoxetine to escitalopram or some other SSRI, I’ve lost count, when all that is happening is that he is numbed, having repeated panic attacks and is unable to sleep. His wife is watching him like a hawk but the strain on both is unbearable and yet – the insistence on SSRIs is beyond comprehension especially when they just don’t have any positive effect on the patient. What has happened to medical training that so many can be so blind to the failure of treatment?
Did research into the dexamethasone suppression test stop at about the time the SSRIs came on board?
I was prescribed an MAOIs: Stelapar.
I was prescribed so many drugs that I feel lucky to not to have lost my intellectual capability or be suffering of any cognitive impairment.
That would destroy me.
Yet another drug that will, I suspect, affect women more than men:
For Immediate Release: July 17, 2012
FDA approves weight-management drug Qsymia
The U.S. Food and Drug Administration today approved Qsymia (phentermine and topiramate extended-release) as an addition to a reduced-calorie diet and exercise for chronic weight management.
The drug is approved for use in adults with a body mass index (BMI) of 30 or greater (obese) or adults with a BMI of 27 or greater (overweight) who have at least one weight-related condition such as high blood pressure (hypertension), type 2 diabetes, or high cholesterol (dyslipidemia).
(Right – and everyone who believes that he/she is overweight isn’t going to latch on to it.)
Qsymia is a combination of two FDA-approved drugs, phentermine and topiramate, in an extended-release formulation. Qsymia must not be used during pregnancy because it can cause harm to a fetus. Data show that a fetus exposed to topiramate, a component of Qsymia, in the first trimester of pregnancy has an increased risk of oral clefts (cleft lip with or without cleft palate). Females of reproductive potential must not be pregnant when starting Qsymia therapy or become pregnant while taking Qsymia. Females of reproductive potential should have a negative pregnancy test before starting Qsymia and every month while using the drug and should use effective contraception consistently while taking Qsymia.
(And accidents never happen.)
Qsymia must not be used in patients with glaucoma or hyperthyroidism.
(I wonder what it’s doing to the HPA system.)
Qsymia can increase heart rate; this drug’s effect on heart rate in patients at high risk for heart attack or stroke is not known.
(If it’s not known why was it approved?)
Therefore, the use of Qsymia in patients with recent (within the last six months) or unstable heart disease or stroke is not recommended. Regular monitoring of heart rate is recommended for all patients taking Qsymia, especially when starting Qsymia or increasing the dose.
(Yet it’s recommended for people with hypertension, hypercholesterolemia and type II diabetes.)
The FDA approved Qsymia with a Risk Evaluation and Mitigation Strategy (REMS), which consists of a Medication Guide advising patients about important safety information and elements to assure safe use that include prescriber training and pharmacy certification. The purpose of the REMS is to educate prescribers and their patients about the increased risk of birth defects associated with first trimester exposure to Qsymia, the need for pregnancy prevention, and the need to discontinue therapy if pregnancy occurs.
(And they’re going to make sure that all prescribers are fully aware of problems and so inform patients?)
Qsymia will only be dispensed through specially certified pharmacies.
(Hmmmmmm….)
Vivus Inc. will be required to conduct 10 postmarketing requirements, including a long-term cardiovascular outcomes trial to assess the effect of Qsymia on the risk for major adverse cardiac events such as heart attack and stroke.
Qsymia is marketed by Vivus Inc. in Mountain View, Calif.
Who, in their right mind, would ingest a substance that is admitted to have such inherent dangers? And does anyone remember Fen-Phen?
Might I suggest that your friend’s husband bins the pills and goes on holiday with his wife, with a few valium.
There comes a point when the pills have to be ditched and trust in the person to ‘behave’ is given back to them.
It is no good switching and switching, one has to stop and take a chance that ‘suicide/something awful’ won’t occur.
Been there, done it, you will be amazed at the transformation – we do not need these nasty, magic mushrooms, fertilised in the organic gardens of a laboratory.
The man needs a chance, and his life needs to be given back to him – take the chance; it is more likely than not that he will improve.
Annie, I wish I could. I’ve tried everything I can think of but his psychiatrist is adamant. His only hope is that his wife is well aware of the dangers and keeps trying also but, as you can imagine, the stress is probably going to do her serious harm. If a medication works, fine but, in the face of repeated failure I am at a loss as to how to deal with the intransigent blindness of the prescribers. Don’t they notice that things aren’t getting better? Thanks for your comment.
I’ve said it a million times, these drugs are hormonal disruptors. They disrupt sexual response, they disrupt sugar metabolism, in some women they disrupt menses. Why is it surprising they cause birth defects?
How could the chronic addition of a hormonal substance to a healthy organism do anything else but cause a disruption in the hormonal balance? Steroids have long been the subject of warnings, these should also be given for SSRIs.
Seroxat/Paxil was deliberately marketed as a drug with known suicidal properties.
The lies that have been told about this drug are heinous and overwhelming and evidence shows this to be so.
If it hadn’t happened to me, I would not have believed it possible and this is the war that has to be fought with the gps and psychiatrists, not just the manufacturers.
The only option is to ditch the drugs, otherwise you are on a hiding to nothing. Our government don’t even realise this is going on.
Hardly a soul/journalist bats an eyelid when Glaxi pay a 3 bn fine. No heads roll, nobody is accountable because morals have no place anymore, it seems.
The Glaxo whistleblower on NaturalNews.com, Blair Hendrick, has made medical history. Because of him, largely, GSK were investigated and found guilty. But not guilty enough, because Seroxat is still being prescribed, they are still making their billions and it has to stop.
You could not make it up???
By the way, Vivus is the company that tried to market a drug for “female sexual dysfunction”. Please watch Orgasm.Inc. I don’t know what one would call a serious documentary that has one howling with laughter one minute, cringing the next and then ready to march in protest. Please try to watch it.
They were very disappointed when the FDA did not approve the drug, but the diagnosis they managed to insert into the DSM survives.
I have read the article by Jimenez-Solem et al. in detail and find some interesting conclusions that have not been mentioned in the present discussion. The authors conclude that the increased risk of malformaions is probably due to an increased rate of ultrasounds during the first days after birth. Several studies (e.g. Bar-Oz et al) have shown that women suffering from depression symptoms tend to have more ultrasounds performed of their baby than the background population. As we well know, septal heart defects are seldom clinically relevant and most of us (up to 30%) live with an atrial septal defect without knowing it. This is an interesting conclusions which could be due to the doctors/mothers increased worry after having taken antidepressants during pregnancy. My final remark is that the absoulte risks are, under any circumstance, very low. We are luckily not confronted with another thalidomide case.
Jens – there is every reason to think this is of thalidomide proportions – see the Herding Women post. There may be 10s of 1000s of miscarriages linked to these drugs and marked increase in voluntary terminations – possibly because of birth defects, possibly simply down to disinhibition. See Healy D, Mangin D, Mintzes B (2010). The ethics of randomized placebo controlled trials of antidepressants with pregnant women. Internat J of Risk and Safety in Medicine 22, 7-16 DOI 10.3233/JRS-2010-0487 – you can get this under the publications button on the blog
My wife has had 7 pregnancies on Effexor. After the birth of our daughter, in 2000, she had anti-depressants pushed on her, without informed consent, for Post-Partum Depression which did not exist. Over the next 8 years she had 7 pregnancies, all while taking Effexor, as this crap is almost impossible to get off once your on it. Each pregnancy had been subsequently more difficult. In retrospect the last 2 pregnancies she exhibited the signs of serotonin syndrome. Every pregnancy she was told by multiple doctors that Effexor was safe in pregnancy.
Here is the outcome of those pregnancies:
1. July 31,2001, miscarriage at 8 weeks gestation with no known cause
2. Cole, born May 12,2002, live birth, full term, persistent Heart murmur, attention issues, would be labeled ADHD
3. Jacob, born August 4, 2004, live birth, full term, extreme low birth weight of 5 pounds, malformed lungs w/ PPHN, diagnosed with Failure to Thrive, with diminished breathing capacity to this day, and dysautonomia. Jacob suffers from central sleep apnea as well as severe night terrors. Jacob at 7 years old has a condition known as encopresis and still can not poop on his own, and has episodes of instantaneous cyanosis and blind rage. He has been assessed for Autism and does show some traits, but has never received a formal diagnosis. Jacob needed to be in the NICU for 10 after birth. After much research on my part I discovered the reason he needed to be in the NICU for 10 days after birth was due to PPHN. He is very small for his age and has learning disabilities. Has been labeled ADHD and likely would also be labeled Oppositional Defiant. Due to these issues we homeschool our children.
4. Andrew, born November 6, 2006, live birth, full term,required resuscitation at birth, heart murmur that subsided after 1st birthday. Attention issues, would also likely be labeled ADHD. Mild learning disability, dyslexia, etc.
5. Matthew, born February 21,2009, live birth, full term, had cyanosis and breathing difficulty from birth that was misdiagnosed, went in to respiratory and subsequent cardiac arrest at just over 1 hour. Autopsy was consistent with a post-mortem diagnosis of Persistent Pulmonary Hypertension caused by venlafaxine toxicity. Subsequent pathology performed by Dr. Hannah Kinney at Harvard. Brain showed excessive neurogenesis in Dentate Gyrus. I am trying to find someone to publish the findings in Matthew’s case to no avail. The “experts” on the case don’t seem to want to publish data that may paint an antidepressant in an unfavorable light.
6. Simon, October 6,2009, miscarriage at 18 weeks gestation, this was during Christiane’s withdrawal from Effexor. No pathology completed.
If one delves deep enough in to the literature surrounding these drugs, each and every one of the effects my children have suffered, were either known to the drug company at the time of marketing the drug, or have come to light since my wife started taking them.
After the miscarriage in 2009, my wife’s menstrual cycle became annovulatory for 9 months. This was confirmed via bloodtests.
7. Daniel who was born on April 1, 2011, was born with the condition known as TGA (Transposition of the Great Arteries),as well as Atrial and Ventricular Septal defects, and a bicuspid Aortic Valve. As you may know, these heart defects have been linked to SSRI/SNRI exposure. The curious part about Daniel is that he was conceived 1 year after Christiane had weaned off of Effexor. I am personally of the belief that either the Effexor has had a genotoxic effect on my wife’s eggs, or there is/was a significant build-up of venlafaxine in her fatty tissue leading to the effect, or thirdly that the Effexor which did wreak havoc on her endocrine system for a very long time even after weaning caused the defect, or some combination of any of the three.