In a recent post Infection Super-Spreaders, I outlined the disturbing publication of an article by Andrea Cipriani and colleagues that gives a green light to prescribing antidepressants to children. Prescribing antidepressants to children could even be part of Precision Psychiatry – a new Oxford University brand to complement Evidence Based Medicine.
Concerned about developments, Jim Gottstein and I wrote to the Bosses of Frontiers – normally we would have written to an editor about something like this but editing is outsourced in the Frontiers business model.
Kamila and Miriam
Kamila Markram C.E.O.
Mirjam Eckert Publishing Director
Frontiers Media SA
Avenue du Tribunal Fédéral 34
1005 Lausanne
Switzerland
editorial.office@frontiersin.org
September 24th 2020
Dear Drs Markram and Eckert
On September 2nd you published an article by Andrea Cipriani and colleagues:
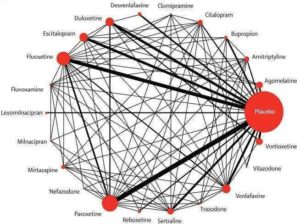
K Boaden, A Tomlinson, S Cortese, A Cipriani. Antidepressants in Children and Adolescents: Meta-Review of Efficacy, Tolerability and Suicidality in Acute Treatment. Frontiers in Psychiatry, doi: 10.3389/fpsyt.2020.00717
For the reputation of Frontiers journals in general, a matter that will be of concern for you, for the safety of anyone taking antidepressants, children in particular, as well as the integrity of the legal system, we think this article should be retracted.
Andrea Cipriani is known personally to one of us and is a very charming man concerned we believe to do his job well. The problem we are raising here is somewhat novel in that we are not criticising the technical methods used in his study – although it can be argued these methods cannot support the conclusions he draws. But there is a more important point.
The general wisdom is that it is a bad idea to introduce a Holocaust comparison into arguments, but we are drawing here on the work of the Jewish sociologist of the Holocaust, Zygmunt Baumann, who was also a leading analyst of technique in general. Applying his insights on technical matters to this instance, we might get the following:
A Frontiers journal could conceivably publish an article on the technical specifications for a sealed rail carriage that allowing for the elimination of defecation and micturition could be used to transport animals several hundred miles ensuring that most if not all of them got there alive – without paying any heed to the fact that the animals to be transported are human.
Dr Cipriani’s article was accepted because some of your reviewers thought the technical specifications were reasonable but the material to which his techniques are applied are a set of ghost-written articles reporting the results of clinical trials to whose data there is no access. Without any consideration of the moral issue of the likely effects on people of publishing his results, the lack of access to data breaches the canons of empirical method,
The consequences of a breach of this kind, which is relatively widespread across medicine, become very clear in the case of trials done with antidepressants in children, where the entire literature has been essentially ghost or company written and where there is no access to the raw data from the relevant trials. In some cases, the data has been destroyed. In one case the mismatch between published articles claiming the trials showed the drugs worked well and are free of problems led New York State to take legal action for fraud against GlaxoSmithKline over paroxetine. In two other cases the US Department of Justice took action against companies for illegal off-label marketing where scientific evidence for their support was lacking, resulting in criminal and civil fines of $3 Billion against GlaxoSmithKline for paroxetine (at the time the largest corporate settlement with the US Dept. of Justice), and $313 million against Forest Laboratories for citalopram in 2012.
It would be a mistake to think that these actions mean the system has managed to spot the bad apples in the barrel – the articles that led to legal actions were not outliers. These trials and articles in fact were likely more ethical than many of the trials done since on which Dr Cipriani and colleagues have depended.
The upshot in the case of antidepressants used for children who are diagnosed with depression is that in fact as one of us has published, there have been 30 trials done, all negative – the greatest concentration of negative trials for any indication ever in human history – yet because of ghost-written publications and articles like Dr Cipriani’s which works from those ghost-written publications, antidepressants may now be the second most commonly taken drugs by teenage girls. The science should make this impossible – it is the ghost-written reporting on this science that has enabled it. Dr Cipriani’s attention was drawn to this, but he chose to ignore the point making him complicit in turning the science inside out, and in so doing he has made Frontiers complicit in this also.
The editor it appears thought there was no room in the Frontiers process for him to draw attention to the issues
There will be deaths as a direct result of this article. In addition to antidepressant scripts escalating rapidly in teenage girls, their suicide rate in the UK has doubled over the past 5 years.
A further concern is the knock-on effect this article will have on legal processes. One of us is a lawyer who has represented people who do not want to take medicines, most often neuroleptics, recommended to them by doctors. These doctors, operating on the basis of a ghost-written literature in which the supposed results of clinical trials are reported which hype the benefit of treatments and hide their harms, have a power they exercise routinely to deprive people of their liberty and force them to take medicines. It turns out that when the real data comes to light, the lack of benefit and the harms such people complain about correspond with what the actual data shows – and the ghost-written literature denies. This situation almost certainly contributes to the widely reported 20-25 year reduction in life expectancy for people diagnosed with serious mental illness.
At the moment, Pontius Pilate-like, the courts and inquests have washed their hands of this issue, but it only takes one judge to put some journal and publication in the firing line.
For the above reasons, we would encourage Frontiers to engage with our concerns about your publication of the Cipriani article.
Yours,
David Healy MD James B. (Jim) Gottstein, Esq.
Professor of Psychiatry Pres. Law Project for Psychiatric Rights
McMaster University, Canada Alaska, USA.
As we composed this letter, the Lancet changed its retraction policy having been shamed by their publication of an article on hydroxychloroquine – see article from Guardian below. The drugs and conditions differ but the key issue the Lancet identified – access to the raw data – is the issue that concerns us and hopefully you.
The Lancet changes editorial policy after hydroxychloroquine Covid study retraction
Tue 22 Sep 2020 05.08 BST
One of the world’s leading medical journals, the Lancet, has reformed its editorial policies following a shocking case of apparent research misconduct involving the study of hydroxychloroquine as a treatment for Covid-19.
In May, the Lancet published a peer-reviewed study about the controversial drug hydroxychloroquine, which concluded Covid-19 patients who received the drug were dying at higher rates and experiencing more heart-related complications than other virus patients.
The large observational study analysed data purported to be from nearly 15,000 patients with Covid-19 who received the drug alone or in combination with antibiotics, comparing this data with 81,000 controls who did not receive the drug.
Questions raised over hydroxychloroquine study which caused WHO to halt trials for Covid-19
This data was recorded by hospitals around the world in a database by a US data analytics company known as “Surgisphere”, the Lancet paper said. The findings prompted the World Health Organization to halt its clinical trials of the drug, given the paper’s findings that it was linked with deaths and complications.
But days after the paper was published, Guardian Australia revealed issues with the Australian data in the study. Figures on the number of Covid-19 deaths and patients in hospital cited by the authors did not match up with official government and health department data. Senior clinicians involved in Covid-19 research told Guardian Australia they had never heard of the Surgisphere database.
Researchers from other countries identified similar issues with the data from their hospitals, and a further Guardian Australia investigation revealed doubts over whether the database used by the study authors even existed. Sapan Desai was a co-author of the paper and founder of the Surgisphere database. Following the revelations, information about Surgisphere was deleted from the internet.
It was also revealed that none of the co-authors of the paper had seen the Surgisphere data for themselves, and they said that Desai did not give them access to it even after questions about the paper were raised by Guardian Australia and the research community. The paper’s co-authors, which included a highly respected vascular surgeon, supported the retraction of the paper and distanced themselves from the data.
While the latest available data shows hydroxychloroquine does not reduce deaths among severely unwell patients in hospital with Covid-19, or reduce illness in those with moderate disease, the higher death rates among those given the drug outlined in the Surgisphere study have never been replicated.
Unreliable data: how doubt snowballed over Covid-19 drug research that swept the world
The publication of the Surgisphere study by the Lancet meant well-controlled studies to definitely determine the drug’s efficacy in preventing or treating the virus were stopped prematurely. Given the drug has been highly politicised by figures such as US president Donald Trump, who has made numerous false claims about its usefulness against Covid-19, rigorous studies into the drug remain important.
World Health Organization studies into hydroxychloroquine resumed following Guardian Australia’s Surgisphere investigation, and the Lancet retracted the Surgipshere paper and vowed to review its publication policy. Such rapid retractions are rare, and followed pressure from the international research community who questioned how the study passed quality control processes.
The new policy, published three months after the study was retracted, requires that more than one author on a paper must directly access and verify the data reported in the manuscript.
“For research articles that are the result of an academic and commercial partnership, one of the authors named as having accessed and verified data must be from the academic team,” the policy states. “In addition, all authors will be asked to sign the author statements form to confirm they had full access to the data reported in their article and accept responsibility for submitting the article for publication.”
One of the questions raised by the publication of the Surgisphere paper was how the paper passed the peer-review process.
The Lancet has made one of the biggest retractions in modern history. How could this happen?
The Lancet has updated its peer-review policy, stating: “Editors will ensure that at least one peer reviewer is knowledgable about the details of the dataset being reported and can understand and comment on its strengths and limitations in relation to the research question being addressed.”
For studies that use very large datasets, such as the Surgisphere dataset, editors will ensure that in addition to statistical peer review, a review from an expert in data science is obtained.
“Finally, we will explicitly ask reviewers if they have concerns about research integrity or publication ethics regarding the manuscript they are reviewing,” the new policy states. The new policy is effective immediately.
https://amp.theguardian.com/world/2020/sep/22/the-lancet-reforms-editorial-policy-after-hydroxychloroquine-covid-study-retraction
Tweet
See new Tweets
Conversation
Pinned Tweet
The Lancet Psychiatry
@TheLancetPsych
In recognition of #PeerRevWeek20 we would like to say thank you to our reviewers for their essential role in maintaining the advancement of science and medicine through helping us to identify the most robust and impactful work #TrustInPeerReview https://hubs.li/H0wB2cg0
GIF
9:04 PM · Sep 21, 2020·Twitter Web App
re the last sentence in DH JG article
The statement that ‘finally we will explicitly ask reviewers if they have concerns….’ is astonishing. That it hasn’t been taken for granted that they would raise concerns is incredible ,that the requirement even needs to said undermines trust in anything reviewers and the journal has published and crucially so been propagated around other academics and whoever else reads their publications. Have reviewers actually been reporting concerns themselves though – and had them ignored or suppressed for whatever reason?
Must say how glad I am that DH and J G have highlighted the fact that people can be forced against their will to take medications which have the potential for causing serious harm and deaths – let alone breach their human rights There is also the fact th people are coerced against their will to take these medications by the overt or covert threat of being incarcerated if they refuse. Where of course long lasting depot injections can be forcibly given.
“By contrast giving treatments open-label slows everything down by leading us up blind alleys while playing roulette with our patients’ lives. I hope this message is clear enough to help sort out this muddled thinking.”
Trial drugs should be used to treat all coronavirus hospital patients, suggests Tony Blair
‘This is not the only politician this year to have expressed opinions regarding unproven therapies and doubtless not the last’
https://www.telegraph.co.uk/news/2020/11/02/trialdrugs-should-used-treat-coronavirus-hospital-patients-suggests/
Meta-Analysis, dubious Data-Sharing Agencies, avoidance-seeking peer-reviewers and death-defying accumulations lead the way like Psychiatric Whatnots and Covid Queens….
https://www.youtube.com/watch?v=6Xw8tkDk5uY&feature=emb_logo
Conclusion and implications for research and policy
https://www.bmj.com/content/351/bmj.h4320
Contrary to the original report by Keller and colleagues, our reanalysis of Study 329 showed no advantage of paroxetine or imipramine over placebo in adolescents with symptoms of depression on any of the prespecified variables. The extent of the clinically significant increases in adverse events in the paroxetine and imipramine arms, including serious, severe, and suicide related adverse events, became apparent only when the data were made available for reanalysis. Researchers and clinicians should recognise the potential biases in published research, including the potential barriers to accurate reporting of harms that we have identified. Regulatory authorities should mandate accessibility of data and protocols.
As with most scientific papers, Keller and colleagues convey an impression that “the data have spoken.” This authoritative stance is possible only in the absence of access to the data. When the data become accessible to others, it becomes clear that scientific authorship is provisional rather than authoritative.
Study 329 was ghost-written, as are all trials of all drugs you might take. The data on what happened to the teenagers in this trial are inaccessible, as are the data from all trials of all drugs you might take. Children of the Cure tells the story of the only pharma company trial where independent researchers have had access to the data and the struggle to publish their findings—a struggle in which medical journals were as difficult as Big Pharma.
https://samizdathealth.org/children-of-the-cure/
Children of the Cure tells the story of the only Medical Study that has two publications in the academic literature—telling precisely the opposite story—and how no one is bothered by this.
Will the dilettantes climb-down, climb-up, conk-out…
Hopefully this will teach someone in the medical-research world a lesson. Never write a paper based on data that you aren’t allowed to see. Sounds drop-dead obvious, doesn’t it? My grandma told me the same thing: Don’t buy a pig in a poke. (Poke being an old hillbilly word for a sack.) And she didn’t have a PhD.
But especially in psychiatry, there has been a pile of recent medical research that’s based on private insurance databases. In particular, the key peer-reviewed articles that allegedly undermined the teen-suicide warnings on antidepressants have all been based on insurance-claims data. “Proprietary” data that no one else can see.
They may be almost as bad as Desai’s work. Granted, the insurance data probably exists. Maybe the authors did the data analysis themselves; maybe the insurance company did it for them. But either way, their work cannot be checked. Anyone who doubts their conclusions will just have to take it on faith: Here is what the data said. (And you’d be hard pressed to find a more polluted form of data!)
It gets worse, however. Even when a paper is based on actual patient research, it is often done by drug-company contractors. The official authors of the paper may never have laid eyes on a single subject, and only seen the official “results” after they’ve been thoroughly massaged by drug-company employees. So I guess we shouldn’t be too surprised when leading experts and major medical journals are taken for a ride by a brash young huckster like Desai with vague but exciting stories about Big Data.
Fire and Brimstone –
I suspect that you could write a brilliant article about ghost writing and kindred behaviour, but this is not it. In my editorial career I have always been suspicious of the pharmaceutical industry – and the question of ghost writing was raised during my editorship of the Lancet – but I have found that medical readers do not relish the fire and brimstone approach. My external reviewer, an academic child psychiatrist, took a similar view. He reckoned that the review should be rewritten, with evidence for and against throughout and minus the direct accusations of immoral behaviour. Whatever you do, I think that you should describe and leave to others to judge.
https://davidhealy.org/neo-culturalism/
Time to Re-Watch the talk in Lyon –
David Healy – GSK 329 Talk – Lyon, October 15th, 2019 (sous-titres ENG et FR)
https://www.youtube.com/watch?time_continue=1&v=ZrYPYlXA0b4&feature=emb_logo
Whatever you think is going on, a smelly figure (breaking bad) with warts and a personality was making the call at the other end of the correspondence. If you were disgusted, you knew what face to put on the voodoo doll as you prepared the pins.
We now have platforms, where we once had bottoms. A topic gets picked by a company like Frontiers who own a platform. They look around for editors to help put together an issue on the now sure-fire topic like antidepressants in children.
But at the moment only one set of interests get heard. …
Clinical Research Facility
Research Themes
Our Work
News
Training & Events
Patients & Public
Blog
You are here: Home / Research Themes / Theme Leads / Profile: Andrea Cipriani
Profile: Andrea Cipriani
NIHR Research Professor, University of Oxford
Cross-cutting theme lead: Informatics / Digital health
Email: andrea.cipriani@psych.ox.ac.uk
Phone: 01865 618228
‘My main research interest is evidence-based mental health and precision psychiatry. My research focuses on the evaluation of pharmacological, psychological and psychosocial interventions, mainly about major depression, bipolar disorder and schizophrenia. I have carried out many systematic reviews, meta-analyses and randomised controlled trials in psychopharmacology, however in the past few years I have been also investigating relevant issues in epidemiological psychiatry and public health, like patterns of drug consumption, risk of serious adverse events (most of all, suicide and deliberate self harm) and implementation of treatment guidelines.’
Andrea Cipriani may be charming as so many of those who who have committed serious wrong doing often are – but if he cannot admit to his errors and retract a publication can we trust there is not or won’t be anything else in the cupboard now or in future. It is the sign of a ‘good’ scientist’ that errors can be admitted and new evidence pursued to correct them.
Along with many others A C’s hospital has set up the Young Persons Advisory Project. to comply with cynical PPI (Patient and Public Involvement) Which, in various guises have fooled more than groups of young people, who thought they could make a difference
As we know these projects give a convenient gloss on research but young people are being duped with fluffy days out and deliberate evasion of a duty to give them accurate information about the scandals historical and current taking place in research. How many will be told about Study 29…
The enthusiasm they start out with is admirable but it is simply corrupt to mis-inform them about all aspects of research.
eg
NeurOx Young People’s Advisory Group
We are a group of young people (between 14 and 18 years old) who work with the Neuroscience, Ethics and Society (NEUROSEC) team to help develop methods for working with young people to better understand their views.
We meet once or twice per term, normally on Saturdays in central Oxford. In between meetings, we keep in touch via email or other online platforms.
Debunking the loss of the Committee on Publication Ethics (COPE) moral compass: conspiracy theory, or genuine cause for concern?
May 2019Eubios journal of Asian and international bioethics: EJAIB 29(3):99-109
Authors:
Jaime A. Teixeira da Silva
67.31Retired / independent
The natural instinct for members of the Committee on Publication Ethics (COPE), which now number almost 12,200, as well as academia, is to assume that this organization works under strict and clearly defined ethical parameters, with a solid vision, and an independent mandate that is not influenced by power, think tanks, or partisan interests. Naturally, whistle-blowing and science shaming are not practices that one would usually associate with an ethics organization like COPE, because they involve ethically and morally questionable practices. Despite this, ethical borders have become blurred between the objectives of Retraction Watch and PubPeer, two self-moderated science watchdogs that rely heavily on these questionable practices, in their efforts to grow and survive, and the values espoused by COPE. A Retraction Watch post, in which the former COPE Chair, Virginia Barbour (2012-2017), made a claim of apparent harassment, is the most striking example of the dangers when collaboration may take place between science-shaming websites, and an ethics organization, COPE. These bonds appear to have been in development for a number of years already, with the inclusion of Elizabeth Wager, the former COPE Chair (2009-2012), as a director of The Center for Science Integrity Inc. (CSI), Retraction Watch’s parent organization. Retraction Watch was financed by, among other groups, the Laura and John Arnold Foundation (LJAF), whose leader, John Arnold, an ex-Enron trader, declared a “war on bad science”, which may naturally include the destruction of aspects of science as well. Retraction Watch embraces several infamous pseudonymous personas under the broad umbrella of freedom of speech, liaising thereby with PubPeer. There is no doubt that errors in the literature need correcting, but this apparent connection with COPE raises questions about the basic ethical foundation of this relationship. Are scientists to embrace this bond between COPE and science watchdogs and pseudonymous whistle-blowers as the new normal in the correction of the scientific literature? This opinion piece puts forward arguments why the author believes that the ethical compass of COPE has become skewed.
– we refer to it as a tsunami –
recovery&renewal Retweeted
Miranda Levy
@mirandalevycopy
Maybe withdrawals from antidepressants aren’t so ‘mild and self-limiting’ after all…
@recover2renew @Altostrata @Mad_In_America @markhoro @wendyburn @rcpsych
‘All hell broke loose’: The truth about coming off of anti-depressants
There is growing evidence that millions are suffering distressing side effects of withdrawal, putting pressure on doctors to act
By Miranda Levy8
November 2020 • 5:00pm
https://www.telegraph.co.uk/health-fitness/mind/broke-loose-truth-coming-anti-depressants/
Today sees the publication of a report in the journal Therapeutic Advances In Psychopharmacology, entitled The Patient Voice on Prescribed Dependence. It finds that 82 per cent of respondents reported the onset of new and unpleasant symptoms on stopping their antidepressants.
Until very recently, the psychiatric “establishment” insisted that withdrawals from antidepressants were “mild and self-limiting over about one week”. In 2018, the Royal College of Psychiatrists sent a letter to a newspaper stating that: “In the vast majority of patients, any unpleasant symptoms… have resolved within two weeks of stopping treatment.”
There was an outcry. Formal complaints were made and signatories – including Dr Wendy Burn, the outgoing President of the Royal College of Psychiatrists – were attacked on social media.
“I started to realise that I was wrong and there was a big problem,” says Dr Burn, who also works as a consultant old age psychiatrist in Leeds.
Why had this taken so long? Part of the problem is a lack of data –
“We tend to work with evidence,” says Dr Burn.
recovery&renewal Retweeted
James Moore
@jf_moore
100-year old woman, prescribed #antidepressants. Why? Because of loneliness and isolation. What’s that going to do for her other than give her adverse effects to deal with on top of everything else? This is no way to respond to COVID isolation
https://metro.co.uk/2020/11/08/doreen-100-wants-to-die-after-eight-months-isolated-in-care-home-13558744/
Royal College of Psychiatrists
@rcpsych
“All the indications are that there is going to be a big surge – we refer to it as a tsunami – of mental illness as a result of the pandemic.” @DrAdrianJames and @AgnesAyton in @Telegraph
https://www.telegraph.co.uk/news/2020/11/08/exclusive-number-people-seeking-help-suicidal-thoughts-has-tripled/
louis appleby
@ProfLAppleby
We need to acknowledge the anxiety people feel in a pandemic without alarming predictions on mental health that could add to risk. So please, no more “tsunami” or “suicide epidemic”.
https://www.thelancet.com/journals/lanpsy/article/PIIS2215-0366(20)30484-3/fulltext
Need for ‘Caution’ …
Anne Guy is a psychotherapist and the co-ordinator for the secretariat of the All-Party Parliamentary Group for Prescribed Drug Dependence.
Marion Brown is the originator of the Scottish Petition.
Stevie Lewis is the originator of the Welsh Petition.
recovery&renewal
@recover2renew
·
This is the newly published #PatientVoice research quoted in @Telegraph
9 November 2020:
The ‘patient voice’: patients who experience antidepressant withdrawal symptoms are often dismissed, or misdiagnosed with relapse, or a new medical condition
Anne Guy, Marion Brown, Stevie Lewis
First Published November 9, 2020
https://journals.sagepub.com/doi/10.1177/2045125320967183#articleShareContainer
Conclusion
We report here on a cohort of patients who were significantly affected by withdrawal from antidepressant (and other prescribed psychotropic) medication and found the response of the health system to their condition inadequate and distressing.
This inadequate response led to misdiagnosis, investigations and further treatment, and caused many respondents to lose faith in the health system and seek help in unregulated peer-led services. It is hoped that lessons can be taken from these experiences and, in line with the recent PHE recommendations, remediations should include: updated guidance about identification of withdrawal symptoms and optimal tapering schedules, which should be widely disseminated and integrated into physician education; dedicated services for those with prescribed drug dependence and withdrawal, including a national help line and appropriately trained staff in primary and secondary care; and an improved feedback mechanism for patients.
In this way, patients currently suffering from dependence and withdrawal issues from antidepressants, and other prescribed psychotropic medication, as well as the large group of patients at risk of these problems, can have their health needs managed appropriately by health services, rather than compounded by them.
Lets hopethe wonderful cmpaignres who have forced this investigation to take place – will demand to knowwhat medications their relatives were put on. My old friend before the pandemic was given antipsychotics without anybody being informed or consenting because she was afraind and distressed after being hospitalised for a heart attack and kept calling out for her sister – it was given deliberately to knock her out. as ‘she was disaturvbing other patients’.. Let’s not pretend there was no intention to allow older frail people to die
Take me to All 4
Menu
22 Oct 2020
Police investigating care home deaths in Scotland
Ciaran Jenkins
Scotland Correspondent
Channel 4 News can reveal that a major police investigation into care home deaths is up and running in Scotland, with officers beginning to gather evidence on all notified care home deaths.
We also understand that some care workers have already spent many hours being questioned by police, which some in the care sector say is causing “extreme distress” among carers trying to prevent a second wave of deaths.
NICE proposes simplifying how medicines and medical …www.nice.org.uk › news › article › nice-proposes-simpl…
9 Oct 2020 — The consultation on the proposals runs until 19 November. A public consultation on the case for change to NICE’s methods for health technology assessment will begin in November 2020. This will be followed by a separate public consultation on the case for change to its processes in February and March 2021.
NICE consults on looking beyond RCTs when evaluating drugs and devices
BMJ 2020; 371 doi: https://doi.org/10.1136/bmj.m4326 (Published 09 November 2020)
Jacqui Wise
The National Institute for Health and Care Excellence (NICE) is proposing placing more emphasis on “real world evidence” when it develops guidance on health technologies, as well as considering additional factors such as the severity of a condition or whether a treatment can reduce health inequalities.
NICE has launched a public consultation that sets out the case for changing the methods it uses to develop guidance on drugs, medical devices, and diagnostics.1 The review was first announced in July 2019 but has been delayed because of covid-19.2
The consultation document says that there should be no change to the general preference for randomised controlled trials (RCTs) to inform estimates of treatment effects but that there should be “an emphasis on the role of a comprehensive evidence base, including non-RCTs and real world evidence.” Real world evidence includes evidence from observational studies, patient registries, electronic health records, and other sources beyond RCTs.
The head of the MHRA which has been so culpable in obscuring, to say the least, adverse effects of ADs and other drugs ,has been on the news bulletins over the last few days to absolutely assure the population in UK that their overseeing of the covid vaccine will be watertight -backed up by the health spokesperson who gives nightly bulletin. They were probably managing the doubt raised by the following advert. Why otherwise such a focus on the potential number of adverse effects expected?
2020/S 207-506291
Contract award notice
Results of the procurement procedure
Short description:
The MHRA urgently seeks an Artificial Intelligence (AI) software tool to process the expected high volume of Covid-19 vaccine Adverse Drug Reaction (ADRs) and ensure that no details from the ADRs’ reaction text are missed.
II.1.6)
Information about lots
This contract is divided into lots: no
II.1.7)
Total value of the procurement (excluding VAT)
Value excluding VAT: 1 500 000.00 GBP
II.2)
Description
II.2.2)
Additional CPV code(s)
48000000 Software package and information systems
II.2.3)
Place of performance
NUTS code: UK UNITED KINGDOM
II.2.4)
Description of the procurement:
The MHRA urgently seeks an Artificial Intelligence (AI) software tool to process the expected high volume of Covid-19 vaccine Adverse Drug Reaction (ADRs) and ensure that no details from the ADRs’ reaction text are missed.
II.2.5)
Award criteria
Price
II.2.11)
Information about options
Options: no
II.2.13)
Information about European Union funds
The procurement is related to a project and/or programme financed by European Union funds: no
II.2.14)
Additional information
Section IV: Procedure
IV.1)
Description
IV.1.1)
Type of procedure
Award of a contract without prior publication of a call for competition in the Official Journal of the European Union in the cases listed below
The procurement falls outside the scope of application of the directive
Explanation:
For reasons of extreme urgency under Regulation 32(2)(c) related to the release of a Covid-19 vaccine MHRA have accelerated the sourcing and implementation of a vaccine specific AI tool.
Strictly necessary — it is not possible to retrofit the MHRA’s legacy systems to handle the volume of ADRs that will be generated by a Covid-19 vaccine. Therefore, if the MHRA does not implement the AI tool, it will be unable to process these ADRs effectively. This will hinder its ability to rapidly identify any potential safety issues with the Covid-19 vaccine and represents a direct threat to patient life and public health.
Reasons of extreme urgency — the MHRA recognises that its planned procurement process for the SafetyConnect programme, including the AI tool, would not have concluded by vaccine launch. Leading to a inability to effectively monitor adverse reactions to a Covid-19 vaccine.
Events unforeseeable — the Covid-19 crisis is novel and developments in the search of a Covid-19 vaccine have not followed any predictable pattern so far.
IV.1.3)
Information about a framework agreement or a dynamic purchasing system
IV.1.8)
Information about the Government Procurement Agreement (GPA)
The procurement is covered by the Government Procurement Agreement: yes
IV.2)
Administrative information
IV.2.8)
Information about termination of dynamic purchasing system
IV.2.9)
Information about termination of call for competition in the form of a prior information notice
Section V: Award of contract
A contract/lot is awarded: yes
V.2)
Award of contract
V.2.1)
Date of conclusion of the contract:
14/09/2020
V.2.2)
Information about tenders
Number of tenders received: 1
The contract has been awarded to a group of economic operators: no
V.2.3)
Name and address of the contractor
Official name: Genpact (UK) Ltd
Town: London
NUTS code: UK UNITED KINGDOM
Country: United Kingdom
The contractor is an SME: no
V.2.4)
Information on value of the contract/lot (excluding VAT)
Total value of the contract/lot: 1 500 000.00 GBP
V.2.5)
Information about subcontracting
Section VI: Complementary information
VI.5)
Date of dispatch of this notice:
19/10/2020
2 of 2
PIMsPlus: Medication Reference Site | Dr. David Healy
Putting the horse before the cart:- This brilliant project PIMs will also probably have the knock on effect of alerting others in elderly peoples’ circles of the need to be vigilant Hope it spreads further and wider quickly when the need has an additional need now Covid is rampant ie many , but certainly not all, will be in very vulnerable positions as Vaccines are being prioritised for older people They will be in fairly powerless situations if they decline, Who knows whether proper assessments of the effects of medications will be carried out. I am pretty cynical about the reason they have been prioritised – huge numbers of people will be being trialled without proper safeguards Or has the government and medicos found a conscience or more likely know the cat is out of the bag so the public won’t keep quiet any longer about deaths of elderly people.
Should Fluvoxaline be on the PIMs list?
‘Filling a Niche’
THURSDAY, Nov. 12, 2020 — The antidepressant drug fluvoxamine — best known by the brand name Luvox — may help prevent serious illness in COVID-19 patients who aren’t yet hospitalized, a new study finds.
The study included 152 patients infected with mild-to-moderate COVID-19. Of those, 80 took fluvoxamine and 72 took a placebo for 15 days.
By the end of that time, none of the patients who took the drug had seen their infection progress to serious illness, compared with six (8.3%) of the patients who took the placebo, according to researchers at Washington University School of Medicine in St. Louis.
“The patients who took fluvoxamine did not develop serious breathing difficulties or require hospitalization for problems with lung function,” said first author Dr. Eric Lenze, professor of psychiatry.
“Most investigational treatments for COVID-19 have been aimed at the very sickest patients, but it’s also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or to have to go to the hospital. Our study suggests fluvoxamine may help fill that niche,” Lenze noted in a university news release.
Fluvoxamine — widely used to treat depression, obsessive-compulsive disorder and social anxiety disorder — is a type of drug called a selective serotonin-reuptake inhibitor (SSRI). This class of drugs also includes medicines such as Prozac, Zoloft and Celexa.
But unlike other SSRIs, fluvoxamine has a strong interaction with a protein called the sigma-1 receptor, which helps regulate the body’s inflammatory response.
“There are several ways this drug might work to help COVID-19 patients, but we think it most likely may be interacting with the sigma-1 receptor to reduce the production of inflammatory molecules,” explained study senior author Dr. Angela Reiersen, associate professor of psychiatry.
“Past research has demonstrated that fluvoxamine can reduce inflammation in animal models of sepsis, and it may be doing something similar in our patients,” she said in the release.
By reducing inflammation, fluvoxamine may prevent a hyperactive immune response in COVID-19 patients. That, in turn, may decrease their risk of serious illness and death, Reiersen said.
“Our goal is to help patients who are initially well enough to be at home and to prevent them from getting sick enough to be hospitalized,” Dr. Caline Mattar, assistant professor of medicine in the Division of Infectious Diseases, said in the release. “What we’ve seen so far suggests that fluvoxamine may be an important tool in achieving that goal.”
The researchers said they plan to begin such a study in the next few weeks and it will include patients from across the United States.
The preliminary study was published online Nov. 12 in the Journal of the American Medical Association.
and
KANSAS CITY LAWYERS | DANGEROUS DRUGS
Luvox (fluvoxamine maleate) is an SSRI (selective serotonin reuptake inhibitor). It has been primarily used to treat Obsessive Compulsive Disorder (OCD) and Social Anxiety Disorder (SAD) . It is marketed under the names:
Luvox
Luvox CR, manufactured by Jazz pharmaceuticals, has recently come under fire from the FDA for it deceptive marketing practices . In 2010, the FDA warned Jazz Pharmaceuticals that a patient brochure for Luvox CR was “false or misleading” because it suggested that Luvox was “safer and more effective than has been demonstrated.”
There is a multitude of adverse effects, many induce the sort of symptoms many older people experience anyway
https://www.medicines.org.uk/emc/product/2416/smpc#gref
This is the only para to warn elderly peopleFluvoxamine 100mg Film-Coated Tablets
Wockhardt UK Ltd
Fluvoxamine 100mg Film-Coated Tablets
Adults When is the cut off for people to be classed as elderly
The recommended dose is 100mg daily. Patients should start on 50 or 100mg, given as a single dose in the evening. Dosage should be reviewed and adjusted if necessary within three to four weeks of initiation of therapy and thereafter as judged clinically appropriate. (Are GPs to be trusted to do this? in home visits/in care homes?)
Adults
Withdrawal symptoms seen on discontinuation of fluvoxamine:
Abrupt discontinuation should be avoided. When stopping treatment with fluvoxamine the dose should be gradually reduced over a period of at least one or two weeks in order to reduce the risk of withdrawal reactions (see sections 4.4 and 4.8). If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose, but at a more gradual rate.
Depression is associated with an increased risk of suicidal thoughts, self-harm and suicide (suicide-related events). This risk persists until significant remission occurs. As improvement may not occur during the first few weeks or more of treatment, patients should be closely monitored until such improvement occurs. It is general clinical experience that the risk of suicide may increase in the early stages of recovery.
Other psychiatric conditions for which fluvoxamine is prescribed can also be associated with an increased risk of suicide-related events. In addition, these conditions may be co-morbid with major depressive disorder. The same precautions observed when treating patients with major depressive disorder should therefore be observed when treating patients with other psychiatric disorders.
A meta-analysis of placebo-controlled clinical trials of antidepressant drugs in adult patients with psychiatric disorders showed an increased risk of suicidal behaviour with antidepressants compared to placebo in patients less than 25 years old.
Geriatric population:
Data in elderly subjects give no indication of clinically significant differences in normal daily dosages compared to younger subjects. However, upward dose titration should be done slower in the elderly, and dosing should always be done with caution.
Elderly people can obviously suffer from any of the adverse effects – including suicidal thoughts (and When is the objectionable label Geriatric going to be dropped altogether ?- )
And of course on Rxisk
DRUG SEARCH
Drug Interaction Checker
fluvoxamine
Search
Showing results for: fluvoxamine
DRUG INTERACTION163
DEPRESSION74
ANXIETY73
DRUG INEFFECTIVE72
SOMNOLENCE67
SEROTONIN SYNDROME66
NAUSEA63
COMPLETED SUICIDE61
PYREXIA59
SUICIDAL IDEATION59
TREMOR58
INSOMNIA55
DIZZINESS54
FATIGUE51
HYPERHIDROSIS49
CONDITION AGGRAVATED45
HEADACHE44
MALAISE43
SUICIDE ATTEMPT42
ASTHENIA41
BLOOD CREATINE PHOSPHOKINASE INCREASED41
AGITATION40
OVERDOSE40
VOMITING39
FALL37
CONVULSION35
DIARRHOEA35
LOSS OF CONSCIOUSNESS35
DYSPNOEA34
PAIN34
CONFUSIONAL STATE32
DRUG TOXICITY31
ELECTROCARDIOGRAM QT PROLONGED31
OBSESSIVE-COMPULSIVE DISORDER31
PNEUMONIA31
RESTLESSNESS31
ALANINE AMINOTRANSFERASE INCREASED30
TOXICITY TO VARIOUS AGENTS30
AGGRESSION29
FEELING ABNORMAL29
NEUROLEPTIC MALIGNANT SYNDROME29
INTENTIONAL OVERDOSE28
CARDIO-RESPIRATORY ARREST27
RASH27
OFF LABEL USE26
WEIGHT INCREASED26
CHEST PAIN25
RHABDOMYOLYSIS25
TACHYCARDIA25
ASPARTATE AMINOTRANSFERASE INCREASED24
DELIRIUM24
DRUG WITHDRAWAL SYNDROME24
IRRITABILITY24
SYNCOPE24
DRUG LEVEL INCREASED23
RENAL FAILURE ACUTE23
AMNESIA22
HYPOTENSION22
MEMORY IMPAIRMENT22
ABNORMAL BEHAVIOUR21
ANAEMIA21
DEPRESSED LEVEL OF CONSCIOUSNESS21
OEDEMA PERIPHERAL21
CHILLS20
CONSTIPATION20
GAIT DISTURBANCE20
AKATHISIA19
ALTERED STATE OF CONSCIOUSNESS19
BACK PAIN19
COMA19
DEATH19
DECREASED APPETITE19
DIABETES MELLITUS19
DISORIENTATION19
DRUG ABUSE19
DYSKINESIA19
HYPERTENSION19
MULTIPLE DRUG OVERDOSE INTENTIONAL19
PARAESTHESIA19
PSYCHOTIC DISORDER19
ARTHRALGIA18
BLOOD LACTATE DEHYDROGENASE INCREASED18
MYALGIA18
MYOCLONUS18
DISTURBANCE IN ATTENTION17
MUSCLE RIGIDITY17
WEIGHT DECREASED17
HYPOAESTHESIA16
HYPOKALAEMIA16
INAPPROPRIATE ANTIDIURETIC HORMONE SECRETION16
PRURITUS16
PULMONARY OEDEMA16
TREATMENT NONCOMPLIANCE16
ABDOMINAL PAIN15
ABDOMINAL PAIN UPPER15
BLOOD PRESSURE DECREASED15
BLOOD PRESSURE INCREASED15
COUGH15
DEHYDRATION15
HEART RATE INCREASED