
There is a core concept shaping the ‘market’ in health, the concept of an assay. Few doctors or patients understand it. This article explains what assays are, how they entered healthcare and the consequences of failing to grasp the role they play.
This post by Harriet Vogt and David Healy is an illustrated version of a peer-reviewed citable academic article Randomized Controlled Assays: A Category Error with Consequences that like the illusions below will look instantly obvious to some but impenetrable to others. Thanks to Bob Whitaker it has also appeared on Mad in America and the article ties closely into the post Has HealthCare Gone Mad.
Before Thalidomide
By 1950, we were starting to get the first new drugs that worked so well no Randomized Controlled Trials (RCTs) were needed to show they worked.
Who said no RCTs were needed? Tony Hill, who in 1947, created RCTs. For Hill conversations between doctors and patients were key. If we are taking a medicine, we depend on someone able to look closely at and listen intently to us. If, instead, we insist on randomization, placebos and double-blinding, Hill said, we’d have lost the plot. RCTs, besides, generate average effects that told him nothing about how to treat the person in front of him.
Louis Lasagna, a big Hill fan, was the leading American cheerleader for RCTs in the 1950s. He had an unfortunate idea. There were still some old drugs around that didn’t work and RCTs might help eliminate these.
This idea went nowhere, until a birth defect crisis triggered by thalidomide, a sleeping pill, struck. In 1962, needing to be seen to respond, politicians adopted Lasagna’s idea of getting industry to prove their drugs work using RCTs. They overlooked an RCT of thalidomide Lasagna had already done, which showed it worked and was free of side effects. Thalidomide had sailed over the hurdle being put in place to prevent a repeat.
After Thalidomide
For two decades after 1962, neither regulators, doctors nor industry understood RCTs. Doctors had been evaluating drugs successfully for a century without RCTs. They knew little about these new trials they were asked to run. FDA bureaucrats knew even less. Industry knew nothing but had an incentive to learn fast.
Doctors ran RCTs for industry, comparing new drugs to old assumed-to-work drugs. When both looked roughly the same, they said “this drug works”. Maybe it did but these RCTs didn’t prove that.
Some RCTs were well designed with important outcomes, such as how many people died on new and old drugs. Trials like this take nearly a decade to run, which is a non-starter for a company hoping to get a drug to market quickly. Worse again in some good RCTs potential industry blockbusters killed more people than the older drugs.
It was time for industry to intervene. They took the running of RCTs out of the hands of academic doctors and gave them to contract research organizations (CROs), whose mission was to get them done quickly. They gave the reporting of trials to medical writing companies whose mission was to get the product in print quickly.
But the most important breakthrough came from the FDA. Paul Leber, the man responsible for approving psychotropic drugs, said “wait a minute, comparing a new and old drug is nuts—neither might work. You have to use a placebo for us to know if your Assay is sensitive enough to pick up the drug’s effects”.
Suddenly, companies were no longer doing RCTs, no longer notionally expected to prove their drugs didn’t work. No longer in the business of demonstrating their drug saved lives or got people back to work. They might be randomizing, and controlling with a placebo, but they were doing Assays—Randomized Controlled Assays (RCAs).
What’s an Assay?
FDA approves Food and Drugs—on the basis of assays. The assay standard for butter is at least 80% butterfat. Chocolate must have over a quota of cocoa solids and under a quota of vegetable oils. When you put a dipstick in your urine to test for glucose, or protein against a standard the dipstick carries, you run an assay. If you want to call a product something, the US Institute for National Standards tells you what the accepted standard is. FDA assays possible butters, chocolates and drugs to see if they meet a standard.
Scientific experiments, like clinical trials, may have standards to avoid mistakes but they don’t operate to a standard any more than a clinical interview has. Standardizing clinical interviews risks disaster.
Science explores, bureaucracy tests. RCTs aim at an external validity that will inform clinical practice. RCAs aim at an internal validity that allows a regulator to tick a box and approve a label for a medicine.
Leber left ‘Randomization’ and ‘Controls’ in place in the new Assays. Seeing them doctors saw RCTs and later Evidence Based Medicine (EBM).
Companies saw meeting a standard agreed with regulators in an Assay. Show us an Assay with a 2 point on average improvement in Hamilton Depression Scale scores as its outcome and we in the FDA can tick the box that lets you call this an antidepressant.
Doctors had the illusion of seeing a Rabbit. Pharma and regulators saw a Duck, but also saw advantages in ensuring doctors do not spot the Ducks. And they don’t.
FDA and companies converged on replacing explorations of drug effects or clinical benefits with tests. Is there a change on standardized interviews (rating scales) or dipsticks? A minimal fall in an average rating scale or dipstick score lets FDA license a company to use words. They can stick words like antipsychotic or statin on drug labels and in adverts.
In 1962, figuring RCTs revealed the truth about a drug, academics had drafted regulations saying FDA could license a drug if there were two positive RCTs. Pretty certain a positive RCT showed a drug worked in the sense of produced a clinical benefit like saving lives, they were absolutely certain two did.
For 20 years after 1962, dealing in RCTs, FDA had preened itself as regulating by science, but it now transitioned back to being a bureaucracy. Looking at the 2 trial criterion, Leber said – “we can tick a box if a company has done 100 Assays and 2 are positive”.
To enable this to happen, a new group of drugs, the selective serotonin reuptake inhibitors (SSRIs) forced regulators into a creative maneuver. SSRIs were never meant to be antidepressants. They are essentially anxiolytics, but anxiolytics were getting a reputation for causing dependence. An antidepressant label offered better marketing opportunities. Convert cases of Valium into cases of Prozac? Of course, we can do that!
Running Assays in people diagnosed as depressed, however, was a problem. Unlike tricyclic antidepressants (TCAs), SSRIs don’t help severe depression (melancholia). SSRI Assays had to be run in mild depression, where it was difficult to beat placebo. Fortunately, however, if the SSRI didn’t beat placebo, the TCA likely didn’t either. FDA could then pronounce this as a failed exercise, where failure meant it lacked assay sensitivity.
There is no such thing as a failed RCT. Demonstrating a drug doesn’t work advances medicine. But assays can fail. If we know a drug works, or a patient has diabetes, but the assay system doesn’t pick it up, we need to change the assay parameters to pick up the known effect.
Dipsticks and rating scales made this a lot easier. It is difficult to say a treatment works if dead bodies are the outcome measure and there are more on treatment. But if the ‘it works’ assay standard is a dipstick or rating scale change, the ‘it works’ box can be ticked even if there are more dead bodies on the new drug. In the last 40 years, almost no set of assays that have led regulators to license a drug have shown lives saved or serious injuries avoided.
Between having to provide only 2 positive assays, being able to write off failed assays, and having a criterion for working that has limited contact with the real world, companies could now get weaker and weaker drugs licensed. A system supposed to eliminate Snake Oil, now encouraged the production of Snake Oils.
Looking at this New Deal, industry saw a beautiful siren. For those on the receiving end of debatably beneficial treatments with hidden hazards, this illusion might show a different face.
Tail Wags Dog
When politicians try to regulate drugs they claim to be regulating industry not medicine. In 1951, as the first really effective drugs came along, they were made prescription-only. Otherwise, companies claimed, they would have to put an entire medical course into a drug label. As a result, we could only get the new miracles from a doctor.
Prescription-only is a police function first introduced for heroin and cocaine in 1914. This is why consulting a doctor can feel like being nice to a policeman.
From industry’s perspective, 1951 also transformed doctors into the consumers of drugs. They consume by putting drugs in our mouth. Rather than having to market to 329 million consumers in America, pharma markets to less than a million. Companies spend vastly more on these medical consumers than any other industry spends on the 329 million, and figure few doctors have a thought in their head that is not put there by their company or their competitors..
There is roughly one cellphone per person in the US. On average every American has 22 prescriptions per year. If each prescription is once monthly and for 5 medicines, this is over 3000 pills per year, some of which cost more than the latest cellphone. Where do company marketing dollars go?
Industry claims that a drug costs several billion dollars to bring to market. This claim has led many to try to establish how much of this is marketing and how much research. Although good figures are hard to come by, the general conclusion is its mostly marketing (to doctors).
It may all be marketing. Big Pharma do little research. They buy small companies who discover new compounds. Their RCAs are commercial exercises, not science, and not research.
Glossy adverts in medical journals or sponsorship of free lunches look like marketing. Those who see healthcare going down the tubes get indignant about these adverts, saying all would be fine if there were no free lunches and doctors were guided by the evidence.
Doctors adamantly insist they are not influenced by adverts. They go by the evidence. Academics even boast that EBM shackles the pharmaceutical industry. Industry take them at their word. If there were no free lunches, glossy adverts or sponsored CME, industry would have to invent them. The indignation these sales (not marketing) techniques create distract from the marketing, the latest ‘apparent’ RCT that the adverts sit beside,
The published RCAs that influence doctors so much and look like RCTs are ghostwritten. Neither the ghostwriters, nor the notional authors, nor the regulators get to see the data from these commercial exercises. Information about what happened in these assays is protected by commercial confidentiality requirements rather than by clinical confidentiality.
When we read that companies will not show our data to anyone, we happily sign consent forms for these commercial exercises. In this way, we put everyone else who is injured by the drug in a state of legal jeopardy because companies can claim nothing like happened in our studies – it’s your anecdote against our evidence.
According to legal and clinical definitions of evidence, there is no evidence in company assays. Legal evidence means someone who can be examined and cross-examined to establish what happened. Did this man, coded as having burns, pour gasoline on himself and set fire to it in a suicide attempt? In a clinical study, people are the evidence.
Not all the people in commercial exercises even exist. Doctors have been jailed for entering non-existent patients into company assays. The people who do exist are increasingly likely to live in Africa or South America. We could manage the evidence issue if we had clinicians who had treated them and could testify. But the authors on the authorship line of any article reporting the results will be American, none of whom will have seen the effects of the company drug on either the far-flung, or even American, patients.
Fraud is a real possibility and it’s less hidden than people might think.
In 2001, a GSK paroxetine Assay in depressed children (Study 329), was published in the best journal in child psychopharmacology, trumpeting paroxetine’s benefits and safety. Doctors rushed to put children on Paxil.
In 2002, applying to the FDA to license Paxil as an antidepressant for children, GSK submitted 3 assays, one being Study 329, stating all 3 were negative. Agreeing the 3 Assays were negative, the FDA agreed to license Paxil for children and not to mention the negative assays in the Paxil label.
A few years later Erick Turner, ex-FDA, published a paper on antidepressant assays done in adults. Almost half of these were negative but like Study 329 many of these negative assays had been published as positive. If the FDA know a significant amount of the published medical literature is fraudulent, why don’t they say anything?
Simple. The FDA tick boxes that let companies make claims. They police advertisements claims but policing the medical literature is not their job.
Whose job is it?
Medical Journal editors see FDA approvals. While they know the articles are ghost-written and have seen companies charged with fraud, they do not view it as their job to police the medical literature either. In 2009, the New England Journal of Misinformation made clear that ensuring the integrity of published data is not their brief.
In 2022, one of us (DH) emailed 300 Professors and other Medical Grandees, who had reviewed articles for NEJM that year. The email drew their attention to the ghostwriting of the vaccine assays, clear evidence that vaccine hazards had disappeared from the published articles and NEJM’s public unwillingness to tackle this. There was no response. A Sodom and Gomorrah for our times?
The Cochrane Collaboration was established in 1992 to gather and sift the published ‘evidence’ so we really knew the truth about the drugs we used. They had an opportunity to say we will only count studies where we have access to the data, but they didn’t grab the opportunity.
In 2004, the evidence that pretty well the entire pediatric antidepressant literature was fake, with GSK sued for fraud, caused Cochrane problems. But Cochrane was wedded to the idea that RCTs produce the truth, whether done by industry or not, and, as by then company studies formed the backbone of EBM, Cochrane held its nerve and continued to collaborate with Industry.
In 2005, Iain Chalmers, the head of Cochrane, testified that ghostwriting is rare and only happens in peripheral journals who publish the dodgy stuff. Richard Horton, editor of the Lancet, said the same thing. The Lancet and NEJM these days likely take more ghostwritten commercial studies that the dodgy peripheral journals. What good would articles in dodgy journals be to industry?
Hypnotized
The upshot of this is doctors are hypnotized. They are now like a bunch of Xtians facing the Bible and the Sacraments and being asked to accept it’s all phony. They’d prefer to be eaten by lions. Company expenditures on hypnotizing these consumers to a greater extent than any other consumers has paid off.
The people we now face may be pleasant, but all too often are automatons. An automatism is another word for a hypnotic reflex.
As regards drugs, doctors have bought into a Magic Bullet ideology. A century ago, Paul Ehrlich, whose research helped create the modern pharmaceutical industry, saw the ideal medicine as a Magic Bullet, a drug that hits a target without causing collateral damage.
Industry now sells doctors on Magic Bullets. If there appears to be collateral damage, doctors increasingly think there was something else wrong with the patient rather than something wrong with the Magic.
Before 1980, the Magic used to lie in the doctor who, among other things s/he did, might give us a potentially risky chemical, a poison, out of which s/he would magically bring a benefit. Now the Magic lies in the pills and if things go wrong a bumbling prescriber is more likely to be blamed.
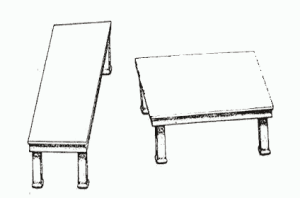
Now that Care has disappeared from healthcare, there are calls to get doctors and patients around the same table and admit the patient’s voice into the discussion. Like motherhood and apple pie, this is obviously a good thing. And some doctors will always agree to get involved in the illusion of communication.
Asked to sit around a table with our doctors, we miss that it’s their table. They will listen to our anecdotes but at the end of the day we all have to go by the evidence. Nothing else would be rational. Your anecdotes explain why you get upset but I can’t start treating you on that basis.
These tabletops are equal in size and shape—the legs give an illusion of difference.
Look more closely at these nice docs. You will see a glassy-eyedness. It’s the hypnosis. It’s not just the docs, non-medical prescribers are affected, and lots of us are also under the influence. It doesn’t pay in this situation to be an educated patient. As with the police, it’s better play dumb. For a conversation, in fact for science, to take place, the spell has to be broken.
Breaking the Hypnotic Spell
Hypnotic spells break if something happens that doesn’t fit the script. Drug-induced adverse events are key to spell-binding or spell-breaking in this case.
If you buy the idea your suicidality on an SSRI is evidence of a bipolar disorder, or your loss of libido, darling, is your depression, you and your doctor become more spell-bound. Standing your ground, though, breaks the spell you are under.
There is another way the spell might break more generally. If our meds work as well and are as safe as pharma claims, non-medical prescribers (NMPs) offer a cheaper delivery system. A switch from doctors to NMPs is happening at pace at the moment, which might wake doctors up and lead them to rediscover how dangerous medicines can be.
For many the natural answer to these problems is more regulation. But regulation has fed this hypnosis. We need to understand regulation’s risk of unintended consequences.
Regulators let companies use words like chocolate or laxative. There are 4 different laxative principles. They can introduce bulk, or fluid, into our bowel, soften our stool, or stimulate bowel contractions. Doctors used to work out which one we needed. The wrong one may put us on the road to treatment resistant constipation. Giving a company the rights to use the word laxative incentivizes their marketing department to ensure all people with constipation get their product. They don’t mind if you get other products also.
Antidepressants illustrate how badly wrong this can go. There are 4 antidepressant principles. Action on the serotonin system gives a serenic effect. Action on noradrenaline energizes. Drugs like mirtazapine improve appetite and sleep; this is a tonic effect. TCAs cure melancholia in a way other antidepressants don’t, perhaps because of anticholinergic euphoriant effect.
The average effect on a Depression Rating Scale that the FDA look at, when reviewing company Assays, obliterates these differences, making it easy to market SSRIs for all depressions.
The problem is that if an SSRI doesn’t help you, it will harm you if only by causing a dependence we don’t know how to treat. If the SSRI fails to work, your doctor likely won’t stop an antidepressant, which FDA says works. Instead, s/he will say “we thought you had a mild depression, but now we see it’s a more serious problem”. This sequence results in treatment resistant depression, a path increasingly likely to lead to medical assistance in dying (MAiD).
Regulation has facilitated a switch from “Therapeutic Principles” to “Magic Bullets”. This hands the narrative to industry, who claim to have made the Magic, which it is now your doctor’s job to prescribe and yours to take. There are no side effects to warn you about. If you get suicidal, your doctor will work out if you’re bipolar or borderline, or if your acne made you depressed and suicidal on Accutane.
Therapeutic Principles, in contrast, typically compensate for a problem rather than cure it and, in so doing, often harm. This sounds less like the Good News of Salvation, but it hands the narrative back to us. We need to think about what we do and monitor what we have done.
Regulation can work against us in other ways. To paraphrase Chuang Tzu (300 B.C), if you have a bunch of little fly-by-night drug companies, it makes sense to regulate and fine them if they cut corners. Rule books make Companies Big by forcing the little guys out of business. If a Giant Pharma then comes along, they will stuff you full of drugs with only one worry that the Red Tape Gag on your mouth isn’t secure enough to hold all of their drugs in.1
Despite all this, we turn now to regulators more than ever before. We get annoyed when they seem to have failed us. What’s going on?
In dangerous situations, we turn to a father figure. It used to be doctors. We saw regulators in a realistic light and did not think bureaucrats could keep us safe. But now that doctors are failing us, regulators have become father figures.
Pharma were quick to spot this. Since 2004, they have been telling us to report adverse effects to the FDA and not to them. We might think that the FDA would more likely agree a company drug has caused our problem. Just the opposite. FDA never agree a drug has caused our problem. Push them on this and FDA say that deciding this is your doctor’s job.
Why Do You Not Believe Me
There are more ways forward. Some that might work in this universe, some for another.
In broken record fashion, the FDA repeat and repeat that it licenses drugs on a benefit risk basis. If there was evidence of lives saved this might be acceptable but there never is.
Only you can make a benefit risk decision. Nicotine can be better than an SSRI for OCD. Only you can tell this if this is the case for you. You also know about the hazards of smoked nicotine, which may be no worse than chronically taken SSRI hazards. You alone can decide if the benefit is worth the risk.
Second, science is on your side. Science is not about figures and statistics. These are tools misused by companies and regulators to browbeat statistically illiterate doctors.
Science aims at consensus on an experiment happening in front of us. When you take a drug you are the experiment. If, invoking a ghostwritten fake literature, your doctor disagrees with you about an adverse event, s/he is not doing science. In contrast to company assays, you give her access to all the data from a drug experiment.
Many hope Artificial Intelligence (A.I.) will solve our healthcare problems. But A.I. can only work on binary options—1 or 0. The effects of drugs on our bodies are never binary but there is a binary option. Should A.I trust a medical literature that only gives the average drug effects from non-existent people? Or should it trust a doctor and patient who, working closely together, have a track record in recognizing when a drug is working or not?
You won’t always be right, but in general if things aren’t working out the key question to post to your doctor is—Why don’t you believe me?
Direct to Consumer
When things go wrong on a drug, or we have a parent or child who needs help, we have skin in the game. This gives many of us a motivation often worth more than medical expertise, especially when armed with the internet and facing medical specialists who know more and more about less and less.
Before 1951, we went to family doctors we trusted to give us good advice. They had to offer us something useful to hang onto our business and even used to visit us at home.
If antipsychotics (tranquilizers) were available over the counter, how many of us would ever have put ourselves on mega-doses of them?
How many of us would increase the dose of an over the counter SSRI if we began feeling bad after the first few pills? Some over the counter antihistamines are SSRIs, which would cause violence, suicide, and sexual dysfunction if we were trapped into obeying doctors’ instructions to keep taking them because they take weeks to work. We manage fine when these SSRIs are normal consumer products.
When companies market to doctors, they have to encourage them to make us diseased, as doctors are only supposed to prescribe for diseases. Freed from the stigma of mental illness, doctors might have tried an SSRI themselves and experiencing genital numbness or Zaps on stopping, might have been more inclined to believe us and more generally insistent on details that should be in drug labels.
Rather than market a tonic to us to improve our appetite or sleep, marketing to doctors means making us depressed. Rather than give us a serenic, which many might feel was not unreasonable to take at times, doctors have to give us an anxiety disorder. Rather than sell us young arteries with a statin or bones with a bisphosphonate, doctors deal in heart attacks, strokes and osteoporosis. If the drugs that companies make money from were over the counter, we would end up less diseased.
Some companies might even opt to make drugs for heart attacks of strokes rather than just aiming for the low hanging cholesterol or blood pressure fruit.
Regulators don’t tell us if this is a good chocolate or if butter is good for us. In any other market consumer associations provide us with that kind of information. When it comes to medicines, doctors should be doing this for us but aren’t and industry have captured the bodies, the guideline makers, who advise doctors on good products (anything new and expensive) and bad products (anything old and cheap). We might be able to set up proper consumer groups if these medicines were over the counter, and doctors might have a role in this working for us.
Drugs that reliably lower lipids or thicken bones would of course add to a wellness, cosmetic or lifestyle market. When drugs like Viagra reliably do something, we can be left to manage them on a Caveat Emptor basis. As with cosmetic surgery, if cosmetic drugs go wrong deciding who’s at fault and suing someone would not pit us against the planet’s biggest corporations.
Surgery is not available on prescription, yet we stick with surgeons because they offer something we cannot get elsewhere. When it comes to tricky medical situations, where the outcomes cannot be reliably predicted, we need doctors who are not automata and can work with us in a developing situation.
Surgeons don’t police us. We don’t need doctors policing us. We need more Obi-wan Kenobis and less Darth Vaders. More willingness to let the force be with us.
After four decades of growing polypharmacy, life expectancies in Western countries are falling. Randomization, whether in Assays or Trials, is never going to help reduce the medications that now burden so many. Now more than ever, we need a relationship based medicine that takes our abilities into account. There is likely to be a lot more force with a doctor who has 100 free research assistants than there ever could be with a doctor burdened by 100 heartsink patients.
Currently, everything conspires to get us to live the lives companies want us to live. Would we be better placed to use company products to live the lives we want to live, if these products were over the counter?
Companies would have a different set of tricks to extract money from us. We who swallow the pills, rather than our doctors, would be the marketing target. But we would have a greater incentive not to swallow baloney. We would have doctors warning us about RCAs masquerading as RCTs. And in the US, we would have 329 million others more conversant with company tricks in normal consumer markets than doctors are in current medical markets.
References
Deloitte Insights. Overcoming Biopharma’s Trust Deficit. Why do people mistrust the biopharma industry—and what to do about it.
This is worth reading for its advocacy of humanizing company CEOs—telling us their reading lists and hobbies—rather than any hint our weirdest ‘market’ might need more radical change.
Healy D. Randomized Controlled Assays and Randomized Controlled Trials. A Category Error with Consequences. Ethical Human Psychology and Psychiatry 2023, 25, 119-134.



Thanks Harriet As well as the content of the blog the thing I think a lot about is the need to get the message out to those who have not come across any activist type work. I do as much as I can from the days of the steam age , doling out paper posters and newsletters until now via the net. But am wondering with your access to people in ‘top’positions whether you are able to inform/educate them by bringing the blogs to the attention of the commissioners at Sussex NHS ? It would be interesting to hear knowing as many of us do how difficult it is to get people in certain positions to listen and how tokenistic or actually obstructive they can be . Is it possible to disseminate the DH and Mad in America to people who use services at the Trust as well as managers and health workers and all those heart=sink doctors who so frustrate and worse those who consult them.
Footnote to explain why I ask. Harriet It would be interesting to hear about your experience.
Jun 2002 – Present · 21 yrs
SussexSussex
I use bespoke research with patient and healthcare professional groups to deliver fundamental insights into their respective needs vis-a-vis product and service delivery.
Strategic Community Ambassador Sussex NHS
Sussex NHS CommissionersSussex
2020 – Present · 4 yrs 2 mos2020 – Present · 4 yrs 2 mos
Working with Sussex NHS Commissioners, applying well honed research and insight skills, to help deliver patient-centric care to the community.
“Breaking the Hypnotic Spell”
Following the ‘Assay’ Trail…
Pierre Kory, MD MPA
@PierreKory
From the start, there was a tsunami of complaints about antidepressants (ie. psychosis, suicide, murder, bipolar, sexual dysfunction).
Lawsuits proved it all was seen in the clinical trials and the FDA covered it up (just like now with the vaccines).
Just How Far Will the FDA Go To Protect A Bad Drug?
What the tragic lessons of the SSRI antidepressants can teach us about the COVID-19 vaccines
A MIDWESTERN DOCTOR
10 DEC 2023
https://www.midwesterndoctor.com/p/just-how-far-will-the-fda-go-to-protect
Putting Lipstick on a Pig
In short, much of the clinical trial industry has evolved into finding elaborate ways to put “lipstick on a pig,” which in my opinion is largely a result of the mass media, medical academics, the medical journals, and the drug regulators being unwilling to call this behavior out and demand the trials be conducted in an accurate manner that will actually predict how the products will perform once they enter the market.
Note: in a recent article, I attempted to illustrate the systemic web of corruption which led to this.
One of the saddest things about this fraud is that doctors are trained to believe all drug side effects (especially from those their specialty regularly prescribes) are “anecdotal” unless there is scientific proof those side effects are real. Yet simultaneously, relatively few of them realize that the “peer-reviewed” articles they rely upon for that proof always censors pharmaceutical side effects. This in turn gives rise to the sad phenomenon of medical gaslighting (which for example we saw throughout the COVID-19 vaccine program).
Kim Witczak
If we don’t start ‘walking down a different street’ nothing will change …
If people are not content for their personal medical history to be used by companies like Palantir, hand in hand with a government which is embroiled in so many scandals it’s hard to keep up with them – it would be wise to use the Right To Opt Out’ of any data sharing,
Pulse Today
NHS England to face legal action over ‘heavily’ redacted Palantir contract
Eliza Parr
19 February 2024
Legal proceedings against NHS England have been launched by law campaigners, in a bid to ‘uncover’ the contents of a controversial contract with tech company Palantir.
Last year, the US tech giant was awarded a seven-year contract worth £330m to deliver NHS England’s new ‘federated data platform’ (FDP).
The BMA and its GP committee have previously expressed concerns over the high value of the contract and particularly how confidential patient data will be used.
In December, the Government published the contract online, but with redactions throughout the documents.
The Good Law Project (GLP), a not-for-profit law organisation, claimed that 417 out of 586 pages have been ‘completely blanked out’ and has started legal proceedings against NHSE.
The organisation said the ‘heavy’ redaction is not only ‘completely unacceptable’ but also ‘unlawful’ as NHS England has not complied with its obligation, as a public body, to state the justification for the redactions.
GLP’s pre-action letter to NHS, sent last week, said: ‘The heavy redactions mean that the public is unable properly to understand or scrutinise the arrangements under the contract, including but not limited to the issue of how personal data will be handled.’
NHS England has been invited to either re-publish the documents unredacted or with redactions that are in line with transparency policy by 26 February.
An NHS spokesperson told Pulse: ‘NHS England has received a pre-action protocol letter and will be responding formally in due course.’
Pulse has approached Palantir for comment.
The Good Law Project is also preparing a separate legal challenge to ‘make sure that every patient can properly protect their privacy’ by using a mechanism to opt out of the FDP.
The organisation has previously raised concerns about Palantir being awarded the FDP contract due to the ‘company’s questionable history’.
Palantir was founded in the US in 2003 and is well known for clients such as the Central Intelligence Agency (CIA) and the United States Department of Defense.
In November, former Cabinet minister David Davis said Palantir is the ‘wrong company’ to lead the FDP, saying that even if it ‘behaved perfectly, nobody would trust it’.
NHS England’s FDP aims to bring together operational data from different organisations to boost collaboration, and will include data such as the number of beds in a hospital, staff rotas, social care places, or the elective waiting list size.
The national commissioner has sought to emphasise that GP data will not be included on the platform at a national level but may be used in local versions if there are already data sharing agreements in place between GPs and the ICBs.
However, earlier this month, the health secretary indicated that GP data could be included on the controversial platform, saying she ‘absolutely’ wants to link up GP data with hospitals.
In September, NHS England announced £2m of funding for an engagement campaign to gather views from patients on how data in their GP record is used – NHS data projects such as the federated data platform will be discussed at these events.This site is intended
Pulse Today
Home News Technology NHS England to face legal action over ‘heavily’ redacted Palantir contract
NHS England to face legal action over ‘heavily’ redacted Palantir contract
Eliza Parr
19 February 2024
Legal proceedings against NHS England have been launched by law campaigners, in a bid to ‘uncover’ the contents of a controversial contract with tech company Palantir.
Last year, the US tech giant was awarded a seven-year contract worth £330m to deliver NHS England’s new ‘federated data platform’ (FDP).
The BMA and its GP committee have previously expressed concerns over the high value of the contract and particularly how confidential patient data will be used.
In December, the Government published the contract online, but with redactions throughout the documents.
The Good Law Project (GLP), a not-for-profit law organisation, claimed that 417 out of 586 pages have been ‘completely blanked out’ and has started legal proceedings against NHSE.
The organisation said the ‘heavy’ redaction is not only ‘completely unacceptable’ but also ‘unlawful’ as NHS England has not complied with its obligation, as a public body, to state the justification for the redactions.
GLP’s pre-action letter to NHS, sent last week, said: ‘The heavy redactions mean that the public is unable properly to understand or scrutinise the arrangements under the contract, including but not limited to the issue of how personal data will be handled.’
NHS England has been invited to either re-publish the documents unredacted or with redactions that are in line with transparency policy by 26 February.
An NHS spokesperson told Pulse: ‘NHS England has received a pre-action protocol letter and will be responding formally in due course.’
Pulse has approached Palantir for comment.
The Good Law Project is also preparing a separate legal challenge to ‘make sure that every patient can properly protect their privacy’ by using a mechanism to opt out of the FDP.
The organisation has previously raised concerns about Palantir being awarded the FDP contract due to the ‘company’s questionable history’.
Palantir was founded in the US in 2003 and is well known for clients such as the Central Intelligence Agency (CIA) and the United States Department of Defense.
In November, former Cabinet minister David Davis said Palantir is the ‘wrong company’ to lead the FDP, saying that even if it ‘behaved perfectly, nobody would trust it’.
NHS England’s FDP aims to bring together operational data from different organisations to boost collaboration, and will include data such as the number of beds in a hospital, staff rotas, social care places, or the elective waiting list size.
The national commissioner has sought to emphasise that GP data will not be included on the platform at a national level but may be used in local versions if there are already data sharing agreements in place between GPs and the ICBs.
However, earlier this month, the health secretary indicated that GP data could be included on the controversial platform, saying she ‘absolutely’ wants to link up GP data with hospitals.
In September, NHS England announced £2m of funding for an engagement campaign to gather views from patients on how data in their GP record is used – NHS data projects such as the federated data platform will be discussed at these events.
RELATED ARTICLES
GP patient records
NHS England removes web page hinting at plans for ‘central database’ of GP patient records
Eliza Parr
14 March 2024
Exclusive NHS England took down a web page which hinted at plans to use GP Connect to create a ‘central database’ of GP-held patient records.
A publicly-available open source page, where NHS Digital stores codes, had previously said it was being used ‘to demonstrate that GP Connect can be used for maintaining a central database of GP data’.
GPs told Pulse they were ‘deeply concerned’ about this, with the chair of the BMA’s GP Committee for England saying it would be a ‘misuse’ of GP Connect, which is intended only for direct patient care.
Sad to see Raanan Gillon ex Editor Journal of Medical Ethics when it was possible to have genuine debates putting his name to this tosh.
The World Medical Association’s revised International Code of Medical Ethics offers a core set of ethical norms for all doctors worldwide to follow, write Raaman Gillon W Parsa-Parsi, Raanan Gillon, and Urban WiesIt to achieve such an outcome that the WMA ICoME international revision workgroup was established,
Extract
A crucial necessity of medical practice is the establishment and maintenance of patients’ trust in their doctors and in the medical profession. To achieve such trust, demonstrated integrity and conformity to a professional code of ethics are key objectives and national medical codes and regulation help to achieve such trust. The increasing intranational and international moral pluralism just mentioned, amplified by globalisation, led the WMA to further expand on its founding mission of cultivating international agreement among its members on a common international code of professional ethics representing a global medical ethos. Such a code should provide reassurance to all patients, regardless of their cultural background, that they can reliably expect professional ethical behaviour, mindsets and adherence to an agreed code of good medical practice from every member of the international medical profession.
It was to achieve such an outcome that the WMA ICoME international revision workgroup was established,
Its all a bit ‘Snipe’ …
louis appleby
@ProfLAppleby
·
Feb 18
Changing light at Llyn Llywenan today.
Great white egret is still around. Plus variety of ducks & waders, including 20+ snipe.
https://fiddaman.blogspot.com/2019/10/uk-suicide-expert-akathisia-can-make.html
Vaughan Gething’s campaign to become the new Welsh first minister
Welsh first minister hopeful …
https://www.theguardian.com/politics/2024/feb/22/vaughan-gething-welsh-first-minister-hopeful-accepted-200k-from-waste-offenders-firm
recovery&renewal reposted
Dee @Deedoherty2· 2h
In case anyone missed it:
A remarkable letter received by Professor David Healy in 2020 from the UK Government in response to Healy’s submission of a summary of the structural issues in the medical system for decades — and which impact us all:
I Recognise these Constraints
April 23, 2020 6 Comments
https://davidhealy.org/i-recognise-these-constraints/
The Perfect Killing Machine claims people in medicine’s highest echelons recognise what killed Stephen O’Neill but figure there’s little they can do about it.
This letter from Frank Atherton is the most extraordinary I’ve received from Government. To see why you need to page down to the letter to which he is responding, a letter that offers his boss, Vaughan Gething, the Health Secretary for Wales, a Martin Luther King opportunity.
Dr Atherton is a decent man. Mr Gething features in an appropriate photo above. The downloadable originals of the correspondence on headed notepaper and with signatures are here – Atherton to Healy, Healy to Gething with prior correspondence between Healy and Gething further down the page. See Stephen’s Voice and SSRIStories.org for more.
2. Since 1990, close to all academic publications about on-patent drugs have been ghost or company written to the extent of portraying negative trials as positive and dangerous treatments as safe.
3. As a consequence, NICE guidelines on the most commonly used treatments in mental healthcare or the conditions they treat are junk.
4. NICE refuses to engage on this issue.
“The structural issues outlined here should shock you.” …
Vaughan Gethin was Health Minister in Wales during D.H’s attempts to engage him.
He is one of the candidates to become Wales’ First Minister. This brings shame on Wales as does the same fobbing off by the Irish and English ministers when refusing to deal with the suicides of young men prescribed SSRI’s
Vaughan Gething is now campaigning to become leader of Wales Senedd (Parliament)
He defends large cash donations from company whose director was convicted of environmental offence twice
VAUGHAN GETHING
WELSH LABOUR
Thursday 22 February 2024 at 6:40pm
Mr Gething has received donations from an environmental group whose director was given a suspended sentence for illegally dumping waste on a conservation site
Welsh Labour leadership contender Vaughan Gething has defended taking £200,000 in donations from a company whose director was convicted of environmental offences.
Current Welsh Government economy minister Mr Gething has received donations from Dauson Environmental Group whose director David Neal was given a suspended prison sentence in 2013 for illegally dumping waste on a conservation site on the Gwent Levels.
At the time, Cardiff Magistrates’ Court heard that toxic liquid had leaked into the water.
His companies Atlantic Recycling and Neal Soil Suppliers were also prosecuted and ordered to pay fines and costs of £202,000.
Atlantic Recycling and Neal Soil Suppliers donated a total of £38,000 to Mr Gething in 2018.
Another one of his companies, Resources Management UK Ltd, recently faced action by Natural Resources Wales (NRW) after complaints about the smell at Withyhedge Landfill in Pembrokeshire. Residents dubbed it a “a stink bomb on steroids”.
Since December 1, 2023, Mr Gething has received three donations totalling £203,000, according to records on the Electoral Commission website.
These include a £100,000 cash donation on 18 December, and another on 11 January, both from Dauson Environmental Group Ltd. Department of Health and Social Care,
For Attention of Matt Hancock On Sept 2nd 2019
The members of the Welsh senedd are not so far taking any action to suspend V G As health minister
He totally ignored the distress and concerns being flagged up by David Healy and the parents of children who had committed suicides after taking AD’s
Just a few snipetts from my Freedom Of Information Requests at the time
Professor David Healy , Bangor University sent you a letter raising concerns about the death and
inquest of Mr O’Neill. Can you please let me know your response”
Your request has been handled under the Freedom of Information Act (FOIA).
The Department does not have a response to the letter dated 2 September. This is because the
Department did not issue a response to that letter, as this was only copied to the Secretary of State
and a reply was not therefore required.
However, further correspondence from Professor Healy to the Secretary of State was received by
the Department on 28th November, and a response to that letter was sent to him on 11th
December. Please note that the Department does not routinely share correspondence sent to or
from members of the public.
Executive Office (Northern Ireland) did not have the information requested.
susanne 20 November 2019
Delivered
Dear Executive Office (Northern Ireland),
Professor David Healy, Bangor University sent you a letter 2nd Sept 2019 raiSng concerns about the inquest held on the death of Mr O’Neill. You have not as yet acknowleged the letter or sent your response. Please let me know what that is..
Yours faithfully,
susanne
https://www.whatdotheyknow.com/request/lack_of_response_to_letter_re_de#outgoing-965122
Link to this
Report
TEO FOI, Executive Office (Northern Ireland) 25 November 2019
Good Morning Susanne
Many thanks for your information request below. I have carried out a check within the Department and at present there is no record of receipt of the letter mentioned from Mr Healy. For this reason no acknowledgement or response has been sent.
Many thanks
Paul Naylor
IMCAB
A5.18
Castle Buildings
Stormont Estate
BT4 3SR
susanne 21 November 2019
Delivered
Dear DoH FOI,
Thank you John
Professor Healy has not received an acknowlegement Can you suggest what to do next ?
susanne
Yours sincerely,
WhatDoTheyKnow
Menu
Lack of response to letter re death of Mr O’Neill FAO Vaughan Gethin
susanne made this Freedom of Information request to Welsh Parliament
This request has been closed to new correspondence. Contact us if you think it should be reopened.
Actions
Welsh Parliament did not have the information requested.
susanne 20 November 2019
Delivered
Dear National Assembly for Wales, For Attention of Vaughan Gethin
Professor David Healy, Bangor University sent you a letter on 2nd Sept raising concerns about the death of and Inquest of Mr O.Oneill Can you please let me know your response
Yours faithfully,
susanne
https://www.whatdotheyknow.com/request/lack_of_response_to_letter_re_de_3#outgoing-965126
Link to this
Report
Information Rights, 21 November 2019
Dear Susanne
Thank you for your email. The Freedom of Information request that you have submitted relates to information not held by the Assembly Commission.
The information you have requested may be held by the office of Vaughan Gething AM, or the Welsh Government if the information relates to the Member’s ministerial responsibilities.
As data controllers in their own right, your request should be sent to them directly for a response. Contact details for Vaughan Gething AM can be found on the Assembly’s website: http://www.senedd.assembly.wales/mgMembe…. Contact details for the Welsh Government can be found on their website: https://gov.wales/contact-welsh-government
With kind regards,
Ryan Bishop
Rheolwr Rhyddid Gwybodaeth, Cynulliad Cenedlaethol Cymru
Freedom of Information Manager, National Assembly for Wales
0200 300 6224
show quoted sections
susanne
https://www.whatdotheyknow.com/request/lack_of_response_to_letter_re_de_2#outgoing-965592
Link to this
Report
DoH FOI, Department of Health (Northern Ireland) 22 November 2019
Susanne
I would suggest he would need to resubmit whatever he sent as I can confirm it has never been received in the Department. I have checked extensively and there is no record of any correspondence being received in the Department of Health from Professor Healy.
John McCann
Information Management Branch
DoH
Tel: 02890 528218
Dr. David Healy
Psychiatrist. Psychopharmacologist. Scientist. Author.
MENU
I Recognise these Constraints
April 23, 2020 6 Comments
The Perfect Killing Machine claims people in medicine’s highest echelons recognise what killed Stephen O’Neill but figure there’s little they can do about it.
This letter from Frank Atherton is the most extraordinary I’ve received from Government. To see why you need to page down to the letter to which he is responding, a letter that offers his boss, Vaughan Gething, the Health Secretary for Wales, a Martin Luther King opportunity.
Dr Atherton is a decent man. Mr Gething features in an appropriate photo above. The downloadable originals of the correspondence on headed notepaper and with signatures are here – Atherton to Healy, Healy to Gething with prior correspondence between Healy and Gething further down the page. See Stephen’s Voice and SSRIStories.org for more.
Dr. David Healy
Psychiatrist. Psychopharmacologist. Scientist. Author.
MENU
The NICE before Christmas
May 6, 2020 15 Comments
In Orders from Nowhere, Vaughan Gething suggested writing to Andrew Dillon of NICE. The correspondence had an Xmas Eve denouement – hence the title.
Above – people in medicine’s highest echelons …..
Vaughan Gethin and the higher echelons in Wales are being exposed by the Covid campaigners.;-
BBC
Gething ex Health minister in Wales automatically deleted messages, Covid inquiry hears
1 hour ago
One of the men hoping to be the next first minister automatically deleted messages from his phone during the pandemic, the Covid inquiry has heard.
A barrister for a bereaved families group said Vaughan Gething used a disappearing messages feature when he was health minister.
The comments came during the first day of the UK inquiry’s Welsh module, sitting in Cardiff.
Mr Gething said last week: “Everything I have got I have provided.”
A senior adviser to the first minister, Jane Runeckles, was also said to have used disappearing messages.
But a Welsh government lawyer denied that WhatsApps were used for making decisions.
Vaughan Gething, who is now economy minister but held the health brief through the pandemic until May 2021, is running against Jeremy Miles to be the next leader of Welsh Labour.
The inquiry heard Health Minister Vaughan Gething turned on disappearing messages
Nia Gowman, barrister for the Covid-19 Bereaved Families for Justice Cymru group, told the hearing that the “limited messages” disclosed to the inquiry showed WhatsApp and texts were used to discuss government business “where they shouldn’t have been”.
“They show Welsh government senior special advisors suspiciously and systematically deleting communications,” she said.
Messages were sent by special advisers to ministers reminding them to “clear out WhatsApp chats once a week”, Ms Gowman said.
“They showed Jane Runeckles, the most senior special advisor for the first minister for Wales, and Vaughan Gething, minister for health, turning on disappearing messages,” she added.
First Minister Mark Drakeford told BBC Wales in January he had used electronic means of communicating “very little” during the pandemic.
But Ms Gowman said Mr Drakeford was regularly using the texting system to discuss policy announcements and seek clarification on the rules.
The counsel to the inquiry itself, Tom Poole, said that hundreds of messages have been disclosed from “numerous Whatsapp groups”.
But he said the inquiry will want to know why messages have been deleted.
The Welsh government’s barrister, Andrew Kinnier, said that “neither Welsh ministers or senior officials used WhatsApps or indeed any other form of informal communication as a substitute for or supplemental means of decision-making”.
After the hearing Plaid Cymru’s spokesperson for health and social care, Mabon ap Gwynfor, claimed “Labour Welsh government’s lies” had been “further exposed on the first day of the UK Covid inquiry”.
“It is quite simply alarming that the most senior special advisers to the Labour Welsh government encouraged each other and others to ‘clear out WhatsApp chat once a week’, and that Vaughan Gething as the health minister at the time turned on disappearing messages,” he said.
The Perfect Killing Machine (David Healy blog) claims people in medicine’s highest echelons recognise what killed Stephen O’Neill but figure there’s little they can do about it.
‘This letter from Frank Atherton is the most extraordinary I’ve received from Government. To see why you need to page down to the letter to which he is responding, a letter that offers his boss, Vaughan Gething, the Health Secretary for Wales, a Martin Luther King opportunity.’
The way the named people above behaved when attempting to get a reponse to suicides an area of Wales mirrors the way they behaved during Covid
Itsgoing to be almost impossible to tease out how much was down to ignorance and panic. Mistakes politicking power games deliberate liestsay the least was handed out to people of Wales. leading to massive breaches of democratic government as being slowly revealed by the Covid enquiry.
It was told that Sir Frank Atherton, chief medical officer for Wales, called the lack of information surrounding different UK restrictions an “omnishambles”.
But many of them are only speaking out now when being forced too.
The tussle between English and Welsh politicians led to more deaths
Sir Frank told the hearing that Wales should have followed England when it made masks law in shops.
Meanwhile a senior civil servant, Dame Shan Morgan, admitted to deleting messages early in the pandemic.
Wales’ chief medical officer Sir Frank Atherton
Figures from the time showed case rates fell in Caerphilly before rising later
The hearing heard an account of the first Welsh government cabinet meeting where Covid was discussed in 24 February 2020 – a month after Sir Frank warned Mr Drakeford of a “significant risk” of Covid coming to Wales.
Minutes claimed that Vaughan Gething, who was health minister at the time, said there had been no imported cases in the UK at that point – there were nine positive cases by 18 February.
The minutes have been disputed by a Welsh government source, who told BBC Wales the document was incorrect in its account of Mr Gething’s remarks.
Problem with that is that civil servants have been implicated with deleting messages..
RCAs – ‘A Category Error With Consequences’
A shout-out to Mark Horowitz; a shout-out to The Perfect Killing Machine; this was the first line :
https://davidhealy.org/i-recognise-these-constraints/
‘The Perfect Killing Machine claims people in medicine’s highest echelons recognise what killed Stephen O’Neill but figure there’s little they can do about it.’
https://www.youtube.com/watch?v=2RnPN0pAbX8
“This was nothing like the Woody Allen-level neurosis that had led me to start them in the first place —
“I realised at that point that I was on this drug not because it was helpful to me, but because when I came off it, I almost died from it — and I was now walking around dependent on it.”
The psychiatrist who got hooked on antidepressants — now, he helps others to quit
Charlotte Lytton
Saturday February 24 2024, 12.01am GMT, The Times
Dr Mark Horowitz welcomed last week’s study in the British Medical Journal showing that exercise could be more than twice as effective at treating depression as antidepressants.
As a training psychiatrist and honorary clinical research fellow at University College London, it’s not just his professional but his personal experience that chimes with the finding that walking or jogging is a better way to alleviate symptoms than drugs (yoga, cycling, strength training and t’ai chi also scored better than antidepressants alone).
Dr Mark Horowitz was suicidal when he withdrew from the the drugs he’d been on for a decade. He thinks they’re overprescribed for difficult patches in life
“This should remind us that a narrow, medicalised view of depression as an illness like any other, requiring medication, is outdated,” he says, “and that a holistic approach to our health, including exercise, is the way of the future.”
Horowitz spent the summer of 2015 running every day, although in his case he was trying to escape the intense panic attacks induced by his attempt to come off the antidepressants he’d then been taking for more than ten years.
“This was nothing like the Woody Allen-level neurosis that had led me to start them in the first place — and I had experienced nothing like it before,” he says. “I felt like I was on the edge of a cliff, or being chased by an animal. I was just hanging on to life at that point.”
When he found himself contemplating suicide, he resigned from his role at the Institute of Psychiatry at King’s College London and, at the age of 33, returned home to his parents in Sydney, Australia, “completely shattered”.
Horowitz’s experience of withdrawal — both as a patient and running a psychotropic drug “deprescribing” clinic at the North East London NHS Foundation Trust — has led him to campaign for curbing the medication taken by a record 8.6 million adults in England in 2022-23, a figure that has almost doubled since 2011 according to the NHS.
Now 41, Horowitz was an unhappy 21-year-old at medical school in Australia when he visited his GP “and, like one in six people in the western world, I was given an antidepressant”. Over the next decade, as he trained in psychiatry and moved to London for his PhD, prescriptions for his daily 20mg dose of escitalopram were repeated without question.
Although he wondered whether the pills were to blame for a growing raft of health complaints (including fatigue, memory loss and poor concentration), “I thought it was the right thing to do,” he says, “like taking your vitamins.”
The trigger to stop was reading an article during his PhD about alarming withdrawal effects that had never been covered at medical school or in his psychiatry training. Historically psychiatric bodies believed that it wasn’t difficult to stop antidepressants and withdrawal was mild and temporary. It is only in the past five years that it has been acknowledged that for long-term users especially, symptoms can be extreme and long-lasting, including vertigo, anxiety, insomnia, panic attacks, brain “zaps” and obsessive thinking, which are sometimes worse than the original symptoms that prompted the prescription.
At the time that Horowitz decided to stop, in 2015, guidelines from Nice, the health watchdog, suggested that doses could be reduced (or tapered) over the course of four weeks, but he found patients in online forums saying it had taken them years, so decided to “split the difference”. He began halving his dose, then halving it again over four months, before ending up having to go back on them to relieve his suicidal thoughts.
“I realised at that point that I was on this drug not because it was helpful to me, but because when I came off it, I almost died from it — and I was now walking around dependent on it.”
That he had taken antidepressants for so long was, Horowitz believes, the reason his withdrawal was so extreme. In 2018 he started tapering for a second time, but much more slowly. Six years on, he is down to his final milligram, and hopes to soon be free of antidepressants completely. “Coming off that medication has given me a second lease on life,” he says.
In 2019 Horowitz and Professor David Taylor, the director of pharmacy and pathology at the Maudsley
Hospital in London (who went through his own “strange and frightening and torturous” withdrawal), co-authored an article in the Lancet Psychiatry journal arguing that tapering should happen far more slowly than official guidelines suggested. It drew global attention and Horowitz received thousands of emails from people requesting his help with coming off their medication “because their doctors didn’t know how”, and “telling me that relatives of theirs had passed away from suicide during withdrawal”.
Many who had sought medical help for their symptoms had been told that they were simply experiencing a relapse of their original mental health condition. “People felt very gaslit by doctors telling them that it wasn’t a real thing.”
In October 2021 Horowitz set up England’s first deprescribing clinic in north London with Joanna Moncrieff, a consultant psychiatrist and professor of psychiatry at UCL, which has so far treated 40 patients but fields referrals for hundreds more a month. “The story is the same,” Horowitz says. “They were put on a drug when something went wrong in their lives” and simply never told to stop.
Some were prescribed antidepressants after a break-up or divorce, others when the challenges of juggling work and young children became overwhelming. Some, like Horowitz, began taking them as early twentysomethings, anxious about their job prospects, and then remained on them well into midlife and beyond. “I don’t think antidepressants are a particularly good treatment for divorce, or not being sure what you want to do with your career.” There has been, he believes, “a huge overmedicalisation of normal human emotion”. Periods of stress or unhappiness are quite normal, Horowitz adds, yet “over the past few decades our society has been taught to see those things as medical illnesses requiring medical solutions” in part due to aggressive marketing from pharmaceutical companies.
Dr Ed Pooley, a GP at JRB Healthcare in Nottingham, suspects that “antidepressants are slightly overprescribed” in many cases, due to long waits for treatments like talking therapy or cognitive behavioural therapy. “Overprescription is therefore done in the absence of other options and is often encouraged by patient request — but is not a decision taken flippantly,” he adds. “All GPs do their best to deprescribe when they can.”
Horowitz maintains that a failure to actively train doctors in deprescribing has led us to the point where almost a quarter of those taking antidepressants do so for more than five years, and questions are growing about the benefits versus risk of long-term use.
“The honest answer is we have no idea,” he says, citing the limited clinical-trial periods — typically a few months, compared with five or more years for drugs such as statins.
Horowitz has now written a handbook with Taylor — The Maudsley Deprescribing Guidelines — setting out step-by-step instructions on how to safely stop all commonly used antidepressants, which he hopes will become required reading for any medic prescribing them, as well as interested members of the public.
Tapering is a multi-stage process, he says. “It generally means months or years and not days or weeks; allowing for individual trial and error — so making a small reduction, monitoring the response and then using that to make a decision about the next reduction — and being especially careful of the last few milligrams, because small doses of the drug have outsized effects.” A seemingly tiny 2mg dose can have almost half the effect of a 20mg dose, he explains, so this last stage typically requires a liquid version of the drug that can be reduced in far smaller amounts.
His ultimate goal is that “different trusts, different GP practices around the country will take up the tools and start to pay more attention to safely stopping these drugs — as much attention as is given to starting them”. Just as airline pilots are taught to both take off and land, “anyone who is trained in prescribing should be trained in deprescribing,” Horowitz says. “Most of these drugs are not intended to become lifelong.”
The Maudsley Deprescribing Guidelines (Wiley-Blackwell), £44.99.
The concept of an Assay – Ill-Gotten Gains…
Power-Grab from Great-Guernsey..
“Evidence-Based Medicine has been hijacked.”
Unfortunately Medicine finds itself standing at crossroads. There are significant seeds of division. The question for you is therefore; Are you going to heal these wounds or empower the irreversible split of healthcare that beckons in an increasingly uncertain future?
Dr Aseem Malhotra reposted
Dr Clare Craig
@ClareCraigPath
An excoriating letter from cardiologist Dr Dean Patterson.
@mottomeneki
Assuming he saw ALL the cases on the island that would equate to 35,000 myo/pericarditis cases in UK and 200,000 in USA.
Dr Dean Patterson, a leading consultant cardiologist in Guernsey and Fellow of the Royal College of Physicians, wrote an extraordinary letter to the CEO of the General Medical Council (GMC) calling for an investigation into unprecedented harms from the COVID-19 vaccines.
https://doctoraseem.com/top-cardiologist-calls-on-gmc-to-investigate-covid-19-vaccine-injuries/
Dr Aseem Malhotra
@DrAseemMalhotra
BREAKING: Eminent Cardiologist & fellow of the Royal College of Physicians Dean Patterson has asked the
@gmcuk
to urgently investigate covid vaccine injuries
‘In my 33 years of medical practice, I have never witnessed such harm from a therapeutic intervention’ ‘The medical establishment appears blind to the harm.
I am extremely concerned that medical practice itself will be irreparably damaged by the fallout from the mishandling of the COVID vaccine side effects’
‘It is my opinion that the side effects being detected are the tip of the iceberg. Healthcare professionals are quite poor at reporting yellow card cases, while the NHS doctors are overburdened and unlikely to spend 30-45 minutes submitting a yellow card incident. This is particularly the case when the same doctors have been indoctrinated with the statement that the covid vaccines are safe and effective, while the evidence for this safety and effectiveness from double blind placebo controlled studies is extremely weak’
It is my opinion that the GMC must not only support whistleblowers like Dr Malhotra, but urgently put in place the following:
1A working group to investigate the Covid 19 vaccine safety. May I suggest you speak with Dr Yvonne Young from the UKHSA and Dr Melissa Heighten (UCL) to invite their views on this matter? I am part of a growing group of doctors who would like to be part of this investigation, as I am sure Dr Malhotra would be.
2A helpline to support doctors afraid of speaking out.
3A helpline to support those who are vaccine injured. Clearly the GMC should seek support from the MHRA and UK gov with funding for this work.
4A panel should be established to open discussion and reporting the above strategy in the media, in a calm unbiased manner to avoid undue stress on the general population and the healthcare system.
In conclusion, I wholeheartedly endorse Dr. Aseem Malhotra and believe that his unwavering commitment to advancing a more patient-centric, evidence-based approach to healthcare makes him a valuable asset to the medical community. I am confident that his contributions in relation to the exposing the truth about the covid 19 vaccine safety, will continue to have a lasting impact on the health and wellbeing of countless individuals. There are many doctors and healthcare professionals who will openly endorse my view, but sadly there are a silent majority who will only endorse my view quietly in private conversation.
Unfortunately Medicine finds itself standing at crossroads. There are significant seeds of division. The question for you is therefore; Are you going to heal these wounds or empower the irreversible split of healthcare that beckons in an increasingly uncertain future?
Senator Ron Johnson
@SenRonJohnson
Happy to repost anytime an eminent doctor is acknowledging COVID vaccine injuries. Thank you Dr. Dean Patterson and Dr. Aseem Malhotra.
Senator Ron Johnson
@SenRonJohnson
·
5h
Throughout the pandemic, dissident voices were silenced. I’ve tried to give those dissident voices a platform to be heard. Here is a link to the full, four-hour event:
https://rumble.com/v4fpw4c-federal-health-agencies-and-the-covid-cartel-what-are-they-hiding.html
Edward Dowd reposted
The Vigilant Fox
@VigilantFox
“You Can’t Hide the Dead Bodies” – Edward Dowd Testifies on the COVID Jabs “One America CEO Scott Davison, in a chamber of commerce meeting, revealed that he had seen 40% excess mortality … for 25 through 64. He said that a 10% increase would be once in a 200-year-flood or three standard deviation event. 40% was off the charts.”
Excess death data is so bad that the UK Office for National Statistics (ONS) is changing its math methodology, which effectively downplays the real number of excess deaths.
https://twitter.com/VigilantFox/status/1762235701532238270
Great Clip…
It’s Health’s Illusions I Recall
Dame June Raine, MHRA’s Chief Executive, will be stepping down in the Autumn following five years in the role
https://www.gov.uk/government/news/mhra-chief-executive-dame-june-raine-to-step-down-later-this-year
Dame June Raine, Chief Executive of the Medicines and Healthcare products Regulatory Agency (MHRA), has today announced that she will be stepping down in the Autumn following five years in the role. She took up the post in August 2019, following a career in medicines regulation.
The Department of Health and Social Care (DHSC) will shortly begin recruitment for a new Chief Executive. Dame June will remain in post until the Autumn to ensure a smooth transition to her successor.
Dame June said:
It has been an enormous privilege to have led the MHRA through a time of change which is unprecedented in UK medical products regulation. I am especially proud that during the last 5 years the Agency has built a new vigilance system, strengthened international and national partnerships, and delivered regulation which has enabled groundbreaking innovation, from gene therapy for sickle cell disease and the world’s first Covid vaccine, to being close to eradicating polio, and from medical device software to AI diagnostics.
It has been an honour to lead an agency which has patient safety as its top priority and makes a difference to the lives of everyone in the UK. While I am stepping back from my MHRA role, I hope still to be involved in contributing to patient safety and public health in other ways.
Prof Graham Cooke, interim chair of the MHRA board, said:
We are all truly thankful to Dame June for her substantial contributions to patients and public health, both in the UK and internationally, throughout her career. Her leadership of the MHRA over the last five years, particularly during the COVID-19 pandemic, has been exceptional.
We are grateful that Dame June has agreed to continue in post while a successor is appointed.
General Sir Nick Carter and Sir Patrick Vallance Join the Tony Blair Institute for Global Change
https://www.institute.global/insights/news/nick-carter-and-patrick-vallance-join-tony-blair-institute-for-global-change
Former Chief of the Defence Staff General Sir Nick Carter and former Government Chief Scientific Adviser Sir Patrick Vallance have joined the Tony Blair Institute for Global Change (TBI) team of expert Strategic Counsellors.
They join former Finnish Prime Minister Sanna Marin in working with colleagues and teams across TBI’s portfolio of countries, advising political leaders globally on their reform programmes.
General Sir Nick Carter (GCB, CBE, DSO) has over 45 years of defence experience, culminating at the top of his profession as the Chief of the Defence Staff for the United Kingdom, where he was the principal military advisor to the Prime Minister, the National Security Council and the Secretary of State for Defence, and as Head of the UK Armed Forces.
As a Strategic Counsellor for Peace and Security, Nick brings leadership experience on matters of national and international security. He provides strategic counsel to TBI’s senior leadership and to the Institute’s global clients at a time of growing geopolitical uncertainty.
Sir Patrick Vallance (KCB, FRS, FRCP) brings significant expertise to TBI’s work on the transformative role science and technology can play for governments and societies around the world. Having previously been Chief Scientific Adviser to the UK Government and President, R&D at GlaxoSmithKline (GSK), Patrick has significant practical experience of government and cutting-edge scientific research.
In his role at the Institute, he will provide strategic advice and guidance to TBI’s global policy work, as well as practical solutions to challenges client governments face.
Tony Blair said:
“I am delighted that General Sir Nick Carter and Sir Patrick Vallance are joining the Institute as Strategic Counsellors. The depth and breadth of expertise they will bring in providing high-level strategic insight and advice within the Institute, and for the governments with which we work, will be invaluable. Our work with countries on peace and security and the opportunities provided by advances in science and technology will be enhanced enormously by their contributions.”
Medicines regulator failed to flag Covid vaccine side effects and must be investigated, say MPs
https://www.telegraph.co.uk/news/2024/02/27/mhra-covid-vaccine-side-effects-mps-all-party-parliamentary/?fbclid=IwAR0GiV2gP234A2Gi0fyRtR_zCDApU_CmolCB_hgGrZe5QKyBL_Kx6OXKQPY
All-party group believe MHRA were aware of heart and clotting issues in February 2021 but did not highlight the problems for several months
The medical regulator failed to sound the alarm over Covid vaccine side effects and should be investigated, MPs have said.
The Medicines and Healthcare products Regulatory Agency (MHRA) is responsible for approving drugs and devices and monitors side effects caused by treatments.
But the all-party parliamentary group (APPG) on pandemic response and recovery, an influential group of MPs, has raised “serious patient safety concerns”. It has claimed that “far from protecting patients” the regulator operates in a way that “puts them at serious risk”.
Some 25 MPs across four parties have written to the health select committee asking for an urgent investigation. In reply, Steve Brine, the health committee chairman, has said an inquiry into patient safety is “very likely”.
In a letter to Mr Brine, the APPG said that there was reason to believe that the MHRA had been aware of post-vaccination heart and clotting issues as early as February 2021, but did not highlight the problems for several months.
Denmark and several other European countries suspended the AstraZeneca vaccine over clotting fears in March 2021, but the MHRA only published safety advice on April 7, by which time 24 million people had been vaccinated.
The watchdog also saw a “signal” for the heart problems myocarditis and pericarditis in February 2021, but did not include the conditions in safety updates until June 2021, MPs said.
“In effect, the MHRA licences medical products as safe knowing it lacks the processes to properly monitor adverse events,” the APPG wrote.
“In the case of Covid-19 vaccines, given the comparatively novel technology and record manufacturing speed, could the MHRA have even properly scrutinised the licensing data or known the short term safety?
“Historically trust and confidence in vaccines and vaccine safety has been high in the UK, but it seems that the experience of the Covid-19 vaccines has undermined this and by association trust in the regulator and the pharmaceutical industry.
“Now more than ever a wide-reaching and in-depth review is needed.”
Underestimates complexities
The group also warned that the MHRA Yellow Card reporting system – which encourages patients and doctors to flag-up medicine side effects – “grossly” underestimates complexities, and in some instances picks up just one in 180 cases of harm.
An analysis by Stockport NHS Foundation Trust found that in the North West of England, 1,058 people had been admitted to hospital with stomach bleeds caused by anticoagulant medication over five years, yet just six Yellow Card reports were made during the period.
Side effects from drugs account for one in every 16 hospital admissions in Britain, and cost the NHS more than £2 billion each year.
But trials are often too small to pick up adverse reactions, particularly when they are driven by rare genetic mutations, meaning it is vital to continue monitoring drugs in the community.
The MHRA recently said it would investigate why blood thinners were causing dangerous side effects in between two and five per cent of patients.
The APPG said it was also concerned that MHRA regulation of medicine was funded by the pharmaceutical industry and said the body had shifted from focusing on scrutiny to trying to help drugs get approved.
‘Watchdog to the enabler’
Dame June Raine, the chief executive of the MHRA, who announced she would be stepping down last week, has previously said the agency was transitioning from “watchdog to the enabler,” a phrase which MPs said warranted its own investigation.
Graham Stringer MP, co-chair of the APPG on pandemic response and recovery, said:
“The MHRA oversees a failing system that is slow to act, causing harm to patients and beset with conflicts of interest.
“We cannot allow it to continue. That’s why we have written to the health select committee calling for an urgent investigation into the MHRA.”
The APPG said that concerns raised directly with the MHRA had been met with “an habitually dismissive and evasive response”.
Dame June said: “We have made significant steps to put patients at the heart of all our work.
“These include incorporating patient views and lived experience into our safety reviews; involving patients in the early stages of planning medicines development and building a new responsive reporting system for patients to tell us about any adverse incidents. We have also led on legislative changes to strengthen surveillance for medical devices and medicines, meaning patient safety is embedded firmly into law.
“Our progress so far in making changes based on meaningful patient involvement gives us a solid base to build upon as we continue on this important journey.”
“We are committed to enabling innovation that brings transformative medical products safely to patients.’’
More to reveal how the Vaughan Gethin previous health minister for Wales behaved during the pandemic (by the way (I put the comments above re Freedom of Information requests to show what we already know really ,but it’s handy to have the proof , That ‘they will always give people the run around, treat people with contempt by with holding info we are entitled to All those were part of chasing up politicians who were in positions to investigate the issue of suicides caused by SSRI’s But who preferred to ignore what DH was saying over and over again. And in my opinion lie about not having received correspondence
The same attitude is revealed by the way Vaughn Gethin and Co. behaved during the pandemic. Bearing in mind somebody with his dodgy history (above) already is one of only two men aplying to ‘lead’ the whole of Wales it will be ‘interesting’ to see how the majority of people in Wales vote.
Extract from Covid Enquiry in Wales
BBC News
Wales’ health minister was unaware there were nine UK Covid cases at the start of the pandemic.
Wales’ chief medical officer Sir Frank Atherton said the Welsh government did not formally discuss Covid for a month, despite having warned First Minister Mark Drakeford that it was likely to arrive in Wales.
He said he told the first minister there was a “significant risk” of Covid coming to Wales on 24 January 2020, but the cabinet discussion did not happened until 25 February.
Sir Frank said Vaughan Gething, Wales’ health minister during the pandemic, was wrong when he told Wales’ first Covid cabinet meeting that there were not any recorded cases in the UK.
Wales’ chief medical officer Sir Frank Atherton
Sir Frank had been at a Cobra meeting on 18 February when it was confirmed there were nine positive cases in the UK.
“We know that Vaughan Gething hadn’t read his pandemic preparedness papers until he needed to revise for this inquiry,” he said. “The fact that he wasn’t aware of Covid’s arrival into the UK when others were preparing is very telling.”