
This post by Peter Selley needs reading in conjunction with Uterine Roulette, Lonesome Heroines and Silent Health. It is about our rights to information rather than about vaccines per se. There is an industry playbook here put in place with SSRI drugs over 30 years ago that needs recognizing and tackling.
GSK (GlaxoSmithKline) are dragging their feet about being transparent with the data from their pivotal phase 3 Respiratory Syncytial Virus (RSV) vaccine trial in pregnant women, terminated on safety grounds in February 2022.
See also:
- Yellow and Other Virus and Vaccine Perils
- A Shot in the Dark for Pregnant People
- Women, Pregnancy, and Clinical Trials
- Another Brick in the Wall
- Women and Children First: The RSV Iceberg
This commercial development, codenamed GRACE, was designed to assess an RSVpreF3 vaccine given in pregnancy to prevent RSV chest infections in babies. It was stopped when an excess of preterm births and neonatal deaths became apparent.
These tragedies must have been “significant” to the women and their families, but, by all standards, the risk was also “statistically significant”.
Stopping a commercial exercise like this was the right thing to do. What was and is wrong is not explaining fully what happened. Rumours of a pre-term births issue were circulating in the investment community as early as March 2022.
Pfizer definitely knew by October; not surprisingly as Eric Simões (see below), lead author of Pfizer’s Phase 2 trial and in charge of their Phase 3 trial, was on the Data Safety Monitoring Board that warned GSK to stop.
The rest of the world had to wait until Freedom of Information requests in November confirmed the danger – a danger that GSK and the wider vaccine community desperately, it seems, wanted kept under wraps. Pre-term births have not been the only problem, there were neonatal deaths as well. And we still don’t have any of the details that might help make sense of this.
The RSVpreF3 vaccine had only been tested in pregnant rats and rabbits – animals whose pregnancies last for one month or less. The only monitoring of the animals’ pregnancies was daily weighing.
There were no Phase 1 intensive safety studies in pregnant women.
In November 2022 I asked medical.information@gsk.com for further information and was told “We are still investigating”.
GSK in Phases
Let’s go back to 2020 when the preliminary Phase 2 trial of this product in pregnant women started.
The trial design recognised the risk of preterm births – so much so that a safety “trigger” was set. But it required about a quarter of births to occur before 37 weeks’ gestation before the trigger would be activated. There was no safety trigger for pre-eclampsia.
This small study showed a slight excess of preterm births in the vaccinated group, but the warning signs were there for disruption to maternal metabolism.
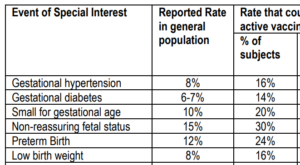
Blood pressure-related problems were reported as an Adverse Event of Special Interest (AESI) more frequently in the two vaccinated groups, compared to the placebo group (far right column).
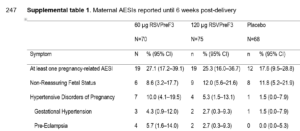
However the study concluded that RSVPreF3 maternal vaccination had an “acceptable safety risk profile”, and waxed lyrical about the massive increase in RSV antibody titres in the babies’ blood samples.
Phase 3
5,330 women were enrolled in this placebo-controlled trial of the vaccine given between 24 and 34 weeks of pregnancy. The only information we have comes from a conference presentation.

GSK’s Ilse Dieussaert (above) drew the short straw and had to present some sketchy findings – See Ilse’s Presentation – about the “Safety Signal” of preterm births at the ReSViNET “Vaccinate the World” congress in Lisbon in February 2023.
Ilse’s presentation was ghost-written by Iona Sima of Akkodis Belgium. She describes an “imbalance” in the proportions of preterm births and neonatal deaths, which is a delicate term to describe the statistically significant danger – in the vaccine group.
This presentation contains the only commercially sensitive information that has been allowed out from this commercial exercise. There is a formal acknowledgment of the problem in GSK’s brief RSVPreF3 OA Sponsor Briefing Document (section 6) for the FDA Hearing that approved the same vaccine for use in people over the age of 60. .
Since then there has been complete radio silence from all RSV researchers about this trial.
Come in Geeta
Dr Geeta K Swamy was the only named author on Ilse’s Lisbon presentation who was not a GSK employee.
Geeta (R) was chair of the INDEPENDENT Data Safety Monitoring Board (DSMB) that made GSK stop their trial. Dr Eric Simões (L), of course, was a member of the same DSMB but also an “author” of the Pfizer RSV Vaccine trial publications. He also ran clinical trials giving AstraZeneca’s nirsevimab (RSV antibody) to newborn babies.
I am at a loss to understand how Geeta could claim to be INDEPENDENT of GSK when she has been employed by them as a clinical trials investigator for other products.
She confesses to being chair of Independent Data Monitoring Committees for GSK (RSV vaccine) and Pfizer (Group B Strep vaccine), Member DSMB National Cancer Institute (HPV vaccine), Member Immunization Expert Group at the American College of Obstetrics and Gynecology. Investigator for clinical trials sponsored by Novavax, GlaxoSmithKline, Regeneron, Pfizer, NIH, and US Centers for Disease Control & Prevention.
The RSV Club is close knit. Almost all the researchers in this field are sponsored by all the vaccine manufacturers and do the peer-review for each other’s articles. Senior members of the club are flown around the world to conferences by Pharma who pay them to sit on DSMBs for their colleagues’ trials.
The report of the GSK Phase 3 trial in older adults of the adjuvanted RSVpreF3 vaccine, ghostwritten by Natalie Denef, of Modis, was published in the NEJM in February 2023. But the outcomes of the pregnant women’s trial, which started and finished before the Older Adult trial remain unpublished.
A Pre-Eclampsia Hypothesis
Let’s go back to part of Ilse’s presentation, which tries to explain the preterm births.
GSK best suggestion is that “additional vaccines” hinting at Covid vaccines might be associated with the excess preterm births particularly in lower income countries. Difficult to know what to make of the Covid vaccine hint – given the abandonment of the Pfizer trial – but perhaps better the Covid vaccine than the Flu or TDAP vaccines – see Uterine Roulette.
This would fit in with what may be a new hypothesis that the preterm births are linked to pre-eclampsia triggered by “inflammation” and the more vaccine shots you have the more inflammation you get – see Uterine Roulette. To gauge what is happening women who have nothing to do with GSK or the RSV industry need the data from this study.
Note added the day after posting
The termination of Pfizer’s Maternal Covid Vaccine Trial in October 2021, after less than 400 or a projected 4000 women were recruited, is shrouded in mystery. See Uterine Roulette. The story put around by Pfizer it seems and promoted by bioethicists who favor empowering women by getting them involved in trials is that by then pretty well everyone had been been vaccinated and it was difficult to recruit.
In early November, Icon who were running the trial on behalf of BioNTech (A Pfizo who is not barking) contacted all research centres telling them that in early August 2021, following discussions with FDA, a revised protocol for the trials included myocarditis and pericarditis as Adverse Events of Special Interest. This with other factors meant that a decision was taken to termination recruitment in late October.
Amazing GRACE?
We still know nothing about maternal safety in terms of preeclampsia/gestational hypertension in the mothers, yet alone about efficacy in the babies in GSK’s RSV GRACE venture.
Now is the time for organisations like ReSViNET, BMGF, and PROMISE, to bite the bullet and discuss the premature births and neonatal deaths issue, and insist that GSK publish the results. The problem is not going to “go away”.
GSK said that neonatal deaths were to be expected in premature babies and had no other cause. There is something inherently wrong about these deaths being dusted under the carpet.
“An in-depth qualitative review of the clinical information available for each neonatal death concluded that the events leading to neonatal death (e.g., very low or low birth weight, sepsis, necrotizing colitis, pneumonia, respiratory distress syndrome, hypoxic-ischemic injury) are commonly observed in preterm-born infants, particularly those who are extremely and very preterm”
That is a whitewash. The suggestion that stopping the trial was all that had to be done to put things right is just not right.
If that number of deaths (13/3496 vs 3/1739 – RR 2.16) had occurred in a trial in a developed country, there would have been an outrage.
Until such time as another patho-physiological explanation is found the assumption must be that vaccination in pregnancy is always dangerous. It is not acceptable to say the cause is having the vaccine in a poorer country.
The charismatic Dr Louis Bont who runs ReSViNET must have something to say about the GSK problem, which is also seen in the Pfizer trials, and the implication for low and middle income countries where infants take the RSV toll. Given he is surrounded by just as many Bont Girls, surely he is no less intrepid than 007 in getting to grips with things that need solving.

ContraConcePTION
The Utrecht Medical Centre has recently announced ConcePTION, with which Louis has links. This aims to set up a registry to make medicines safer for pregnant and breastfeeding women. It is generously sponsored by EFPIA – the European Pharmaceutical Industry.
They use the language of empowerment to good effect but despite asking, ConcePTION has not clarified whether they include maternal vaccines in their remit. Surely they must. But they need to think outside the box and do more than focus on drugs in breast milk as currently billed. In the light of the GSK RSV exercises here and the closely related Pfizer exercises outlined in Uterine Roulette, what about looking closely at placental function and histology?
Pre-eclampsia is a 2,500 year old mystery. Isn’t it time we worked out what is going on?


I think that you have gone a long way to asking ‘What is going on?’ and the Coming of Clean.
Such a captive audience, Pregnant People.
I wonder, if the boot was on the other foot, whether ‘Pregnant People’, would be so accepting if ‘his baby’ inside ‘his womb’ was in danger.
Young people become pregnant, say they bump along at ages 15 to 45, with a few deviations either side. Mostly mid-range, free range.
Young and youngish minds have not been pursued so relentlessly as they are in today’s beatific advances in medicines, rushing headlong in to this captive audience.
Do I take the Covid vaccine.
Do I take an antidepressant.
Do I take an RSV.
It used to be do I smoke, do I drink, what am I supposed to eat.
And, in my case, a call from an hysterical woman blurted ‘ you have a 1 in 64 chance of having a Down’s baby. You have to decide what you want to do’.
Perhaps pregnant women should pop a copy of the BMJ in to their shopping trolley
Others think that notification would have been premature and caused unnecessary anxiety.
https://www.bmj.com/content/383/bmj.p2620
What would an average pregnant person know of GSK (OFF) and Pfizer (ON) RSV, clinical trials, ghostwriting, have they heard of Albert.
Would your average GP say, I should point out that GSK had difficulties…
Albert Bourla
@AlbertBourla
Three years ago today, we were able to share with the world the joyous news that our #COVID19 vaccine was highly effective in preventing disease. It was one of the best moments of my career. We could not have reached this point without the extraordinary efforts of our talented and dedicated @Pfizer colleagues and
@BioNTech_Group partners. This photo was taken moments after we received the good news from our R&D team, and you can see the smiles through our masks. As Aristotle said, “Our problem is not that we aim too high, and we miss. Our problem is that we aim too low and hit.” We reached one moonshot with our #COVID19 vaccine, and now we want to reach more—because our patients are counting on us.
https://twitter.com/AlbertBourla/status/1722657412551721455
You can see the smiles through our masks…
Our patients are counting on us.
I know no other group of people who do as much research as pregnant women – no matter what age. They aren’t push-overs. Hanging together they can sort a lot out – hanging singly they or their babies risk dying.
D
There is a nice summary (in English) of a review of Abrysvo – Pfizer’s RSV vaccine – in pregnancy in the respected French journal Prescrire.
https://www.prescrire.org/fr/3/31/67471/0/NewsDetails.aspx
On it
“One infant in the vaccine group died due to very preterm complications (birth at 27 weeks’ gestation), although a link to the vaccine could not be excluded. An increase in preterm births was also observed in an immunogenicity assay in 579 women.”
and Off it…
An RSV Vaccine During Pregnancy
The CDC recently recommended an RSV vaccine for pregnant women
PAUL OFFIT
https://pauloffit.substack.com/p/an-rsv-vaccine-during-pregnancy
The pivotal trial of Pfizer’s RSV vaccine included…..
When the FDA and CDC considered this vaccine, safety was an issue. That’s because Pfizer wasn’t the only company making an RSV vaccine for pregnant women. GlaxoSmithKline (GSK) also made a vaccine that was virtually identical to Pfizer’s (i.e., 120ug of the RSV F protein, unadjuvanted). GSK, however, abandoned their maternal RSV vaccine because of a concern about an increase in premature births in the vaccinated group.
When two companies make virtually identical vaccines, and one of them has a problem, two possibilities exist. Either both have a problem—and one of them hasn’t realized it yet—or neither have a problem. Indeed, although it wasn’t statistically significant, 33 more cases of prematurity occurred in Pfizer’s vaccine group than the placebo group. All cases occurred more than one month after receiving the vaccine. Although this difference wasn’t statistically significant, it was worrisome enough to the FDA that although Pfizer had asked for a license to administer the vaccine between 24 and 36 weeks of gestation, the license was granted only for 32 to 36 weeks of gestation. By limiting licensure to third trimester use, the FDA lessened the impact of prematurity should it be found in post-licensure studies.
Expectant parents deciding about Pfizer’s vaccine should consider the following ~~~~~
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-22/06-Mat-Peds-Fleming-Dutra-508.pdf
Trial of a similar GSK maternal RSV vaccine (stabilized prefusion F protein vaccine without an adjuvant) was halted due to an imbalance of preterm births with higher numbers in the vaccine vs placebo group
Reason for the imbalance remains unclear
No serious adverse events in infants were considered by the investigators to be related to the vaccine. For infant deaths in the RSVpreF group, the FDA agreed with the investigator’s conclusions for 4 out of 5 of the infant deaths; however, for 1 case of extreme prematurity in an infant born to an 18-year-old mother at 10 days after vaccination who died from prematurity-related complications, FDA was unable to exclude the possibility of the extreme prematurity and subsequent death being related to receipt of the investigational product. No non-fatal SAEs in infant participants were considered related to maternal vaccination by FDA
Ilse Dieussaert is not even a physician. People like her are given lofty positions in industry without any major clinical expertise. I note that her presentation as you said was ghostwritten. Probably very well paid as well.
Giggling Ilse, photo, with description
Ilse
Director, Lead Vaccine Development Lead, Maternal Immunization, Slaoui Centre for Vaccine Research
She also wrote that GSK will remove Slaoui’s name from its research and development center in Rockville, Maryland. It had been called The Slaoui Center for Vaccines Research.
GSK fires Trump Covid vaccine chief Moncef Slaoui for ‘substantiated’ sexual harassment claims
https://www.cnbc.com/2021/03/24/moncef-slaoui-fired-from-galvani-bioelectronics-board-of-directors-over-sexual-harassment-allegations.html
Additionally, GSK announced that it would rename its Slaoui Center for Vaccines Research in Rockville, Maryland.
https://www.fiercepharma.com/pharma/slaoui-agrees-return-386-million-glaxosmithkline-after-his-firing-sexual-harassment
Maybe she had recollections of Moncef…