
Prozac’s commercial success after its launch in 1987 spurred SmithKline Beecham, Pfizer, and others to bring Paxil (Seroxat, Deroxat, Aropax), Zoloft (Lustral), and other Selective serotonin reuptake inhibitors (SSRIs) to market.
En route there was the tricky problem of managing what was recognized within companies by the early 1980s but denied in public, namely, that these drugs could cause agitation up to an including suicidality. There was an even trickier problem – managing the data on suicidal events from the clinical trials done to bring these drugs to market.
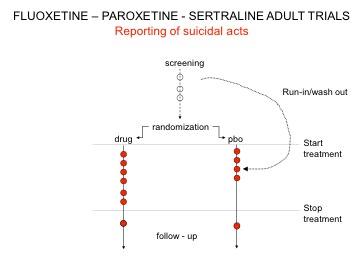
Lilly blazed a trail with suicidal acts on Prozac (see Drug companies use studies the way a drunk uses a lamppost). The strategies included discounting some suicidal acts on the basis that these were not genuine, including under the heading of placebo events that happened after withdrawal from Prozac, but in particular taking events before the trial proper had started when people had their prior treatment discontinued and before they began the double blind phase of the study. These events were filed under the heading of placebo on the basis that the patients weren’t actually on active drug at the time.
SmithKline filed their submission on Paxil with the FDA in 1989 shortly before concerns about suicides on Prozac became public in 1990 – see Table 1.
Table 1
|
Original 1989 Paxil Figures |
||||
|
Drug |
Patients |
Suicides |
Suicidal Acts |
% Suicides & Suicidal Acts |
|
Paxil Comparator Placebo Washout/Run In |
2,963 1151 554 |
5 3 0 2 |
40 12 1 2 |
1.52% 1.30% 0.20% |
Once suicide on Prozac became an issue, these figures posed an even bigger problem than they did to begin with. But somewhere in 1990, the reviewer of the Paxil application, Martin Brecher, developed the impression that FDA had decided the issue of suicide on antidepressants was: “not .. a real issue, but rather as a public relations problem” (Brecher Doc). He invited SmithKline to submit another set of figures.
In the revised set of figures, under the heading of placebo there are two completed suicides and five more suicidal acts that had not been there before. The two suicides and two more suicidal acts came from the washout phase of studies — just as Lilly’s suicide had been.
Table 2
|
Revised 1990 Paxil Figures |
||||
|
Drug |
Patients |
Suicides |
Suicidal Acts |
% Suicides & Suicidal Acts |
|
Paxil Comparator Placebo |
2,963 1151 554 |
5 3 2 |
40 12 6 |
1.52% 1.30% 1.44% |
Was Brecher alone in his understanding of the issues as a public relations matter? When there were hearings on antidepressants and suicide in September 1991, the FDA could have brought the data on suicides and suicidal acts on both Paxil and Zoloft into the frame but didn’t. Why not?
Adding the original Prozac, Paxil, and Zoloft figures together as of 1991 would have shown an absolutely conclusive picture of suicide risk on this group of drugs. Cynics might say that it’s clear why FDA didn’t permit these extra data in: the hazard would have killed the SSRIs. But at the same time, FDA licensed Clozapine despite the lethal risk of agranulocytosis it posed, and a case can be made that this risk helped create a perception of greater efficacy for Clozapine and may have increased sales and profitability.
If a class-wide labeling had been applied to all antidepressants, no individual drug would have been disadvantaged, and the market would likely have developed in almost exactly the same way as it did. We know that senior FDA figures considered the option of a class-wide warning. This seems to put them into a position of being aware that the risks did not come from just one drug and seems to position them in a management of perceptions domain.
We need to be told what happened. The senior figures at the FDA at the time and later were Russell Katz, Bob Temple, and Paul Leber. At the FDA hearings in September 1991 on antidepressants and suicide, FDA officials and others put forward the view that warnings might be the right thing for those vulnerable to the risks posed by SSRIs but the wrong thing for the many who would be deterred from seeking treatment by prominent warnings.
Jonathan Cole one of the authors of the original paper on Prozac and suicide risk fingered Bill Potter (then of the National Institute of Mental Health (NIMH) and shortly afterwards an employee of Lilly’s) as the person who put forward the argument that warnings might do more harm than good, but it seems clear that others put it forward also.
In the background are the conspiracy theories. People note that George H Bush had been on the board of Lilly, that other Lilly officers involved in shaping some of the public relations messages later had prominent political positions in Washington, or as state governors. But this doesn’t readily account for the failure of regulators in Britain, Germany, and elsewhere to pick up the problem.
Why didn’t the bureaucrats bark? As we shall see in later posts, bureaucrats from the regulatory apparatus seem to miss an astonishing amount of what is going on.
How was/is international co-ordination of positions on these risks achieved? In the midst of the pediatric antidepressants and suicide problems, Bob Temple of the FDA said there was no liaison between the FDA and other regulators, such as Britain’s drug regulator – the MHRA. Are we talking about no formal liaison, no minuted liaison, or absolutely no liaison?
It also doesn’t account for the failure of medical or other academics to pick up the problem. These weren’t completely hidden data. Lilly’s ghost suicide appears in the footnotes of a BMJ article. SmithKlines’ ghost suicides and suicidal acts can be tracked down from Martin Brecher’s Safety Review of Paxil, which is a public domain document.
Figure 1 shows what’s going on. This breached regulations. Many looking at it use words like Fraud. Ten years later the regulators told companies – quietly – they could not do this anymore. But by then other strategies had been working out to handling the public relations issues.
Figure 1: Ghost Suicides and Suicidal Acts
Je suggere la creation d’un obrtrvaeoise national des non-sujets, qui serait charge du signalement precoce de ce genre d’enfumage, et qui permettrait de garder du temps de cerveau disponible pour les vrais questions.
I haven’t seen this mentioned often but I inherited one patient – say “inherited because I was NOT responsible for the prescription – who developed a pseudolymphoma from Prozac. She was sent to me because of the cutaneous lymphoma that disappeared as soon as the drug washed out.