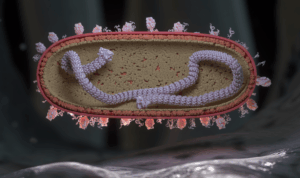
An illustration of the respiratory syncytial virus (RSV) in cross-section landing on a respiratory endothelial cell in humans. A nuclear protein core capped by large L proteins is depicted with single-stranded RNA (colored purple). The virion exterior is populated by F and G proteins, while its interior is lined by M2-1 and M proteins. G proteins are shown as tangled tubular structures, and F proteins are represented as structures resembling drumsticks.
Credit: NIAID CC BY 2.0 Human Respiratory Syncytial Virus (RSV) (52501736052).jpg
What Have We Here
Over the last 3 years RxISK.org and DavidHealy.org have run a series of RSV (Respiratory Syncytial Virus) posts, listed below. We (Peter Selley and David Healy) got into this thanks to some marvellous and continuing sleuthing by Peter Selley.
Yellow and Other Virus and Vaccine Perils — August 8, 2022
A Shot in the Dark for Pregnant People — May 7, 2023
Women, Pregnancy, and Clinical Trials — June 2, 2023
Another Brick in the Wall — September 11, 2023
Women and Children First: The RSV Iceberg — November 16, 2023
Coming Clean on Neonatal Deaths — December 12, 2023
For Every Matter under Heaven, there is a Season — March 18, 2024
The Once and Future Immunity — April 14, 2024
It is Hard for Thee to Kick Against the Pricks — October 21, 2024
The Respiratory Syncytial Virus Challenge — February 16, 2025
The Miracle of Artificial Intelligence — August 10, 2025
From Just Say NO to Getting to YES — September 1, 2025
The Miracle of Articial Intelligence brings RSV in by the back door. Just Say develops the role A.I. is increasingly likely to play in the RSV story.
The State of Play
The licenced or en route to being products licensed by FDA for preventing respiratory syncytial virus (RSV) associated disease in infants, or the elderly. These are approves as Just Say No will illustrate on a specific effect on the RSV virus rather than on the basis of overall benefits to mothers, fetuses, infants or the elderly.
- Abrysvo® maternal – unsettled concerns the consequences for mothers and babies
- Arexvy® maternal – withdrawn because of consequences in mothers and babies but licensed for older folk.
- mRESVIA® maternal (mRNA) – trials in progress
- mRESVIA® infants (mRNA) – withdrawn as linked to severe respiratory illness
- Beyfortus® infants – uncertainty about safety
- Enflonsia® infants – uncertainty about safety
- Synagis® (palivizumab) infants – the first monoclonal for RSV, sidelined because no longer a money-maker.
Known Unknowns
Neither we nor anyone else seems to know:
- If these immunisations reduce illnesses, and deaths from all conditions?
- If massive levels of antibodies in babies interfere with RSV testing from nasal swabs?
- How many immunised babies are hospitalized for chest infections in their second RSV season?
- Whether 105 mg of clesrovimab is a dangerously big dose for a preterm baby?
- What causes Vaccine Associated Enhance Respiratory Disease (VAERD) in RSV-naïve babies?
- Whether we should worry about antidrug antibodies to these monoclonals?
- Whether we should worry about the development of resistant viruses?
- Whether the RSV campaign has interfered with uptake of more important maternal and child vaccines?
- Whether immunisation is as safe or effective as immunity from a natural infection allied to maternally derived immunity?
- Why these immunisation programs were introduced globally so rapidly?
- How rapid “Fast Track” assessments by regulators were justified?
RSVP
We are interested to hear from anyone who can shed any light on any of the above.
That is a load of very interesting questions.
Why these immunisation programs were introduced globally so rapidly?
CDC – RSV
https://www.cdc.gov/rsv/index.html
As Robert Kennedy Jr. is pulling apart the CDC, by ousting new director, Susan Moneraz, and appointing a new CDC Acting Director, Jim O’Neill.
As Robert Malone ,has said previously, he is blaming the CDC for withholding information, which only gave them a day to consider the information before the vote. He voted in favour. He wrote on his Malone news that he would never trust the CDC again.
Retsef Levi knew better, he voted against.
There are literally reams of media, advocating RSV:
https://www.gov.uk/government/publications/respiratory-syncytial-virus-rsv-symptoms-transmission-prevention-treatment/respiratory-syncytial-virus-rsv-symptoms-transmission-prevention-treatment
Let’s choose Ireland:
https://her.ie/health/hse-extends-rsv-winter-programme-644898
‘Not only will the RSV programme help protect infants, but it also lifts immense pressure from the healthcare service during the already busy winter months.’
‘a jab into the baby’s leg.’
RSV getting a ‘leg-up’ from all and sundry.
Ground Control to Major Tom
Your circuit’s dead, there’s something wrong
If Major Tom had made it back to Earth he would probably been eligible for RSV vaccination. Previously in this blog we haven’t discussed the use of RSV vaccines (Abrysvo, Arexvy and mRESVIA) in the elderly.
By happy coincidence two papers were simultaneously published about this – one in NEJM the other in JAMA. They used the same data and were written by the same team. The DAN-RSV Trial, comparing vaccine to no vaccine, was sponsored by Pfizer although no Ghost Authors are acknowledged. The trial was conducted by the European LifeCare Group: motto – “Nothing saves lives like vaccinations” which is probably true.
These papers claim that RSV vaccines prevent cardiovascular hospitalizations and reduce the incidence of hospitalization for RSV-related respiratory tract disease in older adults.
The only problem is that more vaccinated participants err… died.
Whilst all-cause hospitalisations were reduced by 2%, all-cause deaths increased by 21.7%.
For those with preexisting cardiovascular disease 74/14377 vaccinated participants died, compared to 47/14285 in the group that were left alone – an increase of 56.5% (133.5 – 8.5).
These data can be found in the on-line supplements.
Can you hear me, Major Tom?
It’s quite possible there isn’t a note re ghostwriters in small print at the end because A.I. has drafted the manuscript – as Albert Bourla said in his autobiography back in 2021 would be happening very soon.
The Supplement is well worth looking at in addition to reading. Its full of bold print positive results for hospitalizations linked to confirmed RSV lower tract respiratory infections – all of which convincingly make the case for the vaccine. Then on the last page, in much smaller print and right down at the bottom of a set of small print mostly positive findings are the findings on all cause hospitalizations – no benefit – and all cause deaths – meet God sooner rather than later.
It’s too late for Jorge Mario Bergoglio (Pope Francis) but perhaps Major Tom should remain a Doubting Thomas?
David
The JAMA advertorial is “open access” but the NEJM one is behind a paywall (although you can sign up to have access to 2 articles a month for free).
https://jamanetwork.com/journals/jama/fullarticle/2838491
https://www.nejm.org/doi/full/10.1056/NEJMoa2509810
The small print at the end of the JAMA article does not list ghosts. In this case, there may be no ghosts or medical writers although there is a long list of Pfizer employees linked to the study. The small print says Pfizer Inc made no contribution to the running or analyses or interpretation of the data but Pfizer Inc is legally a person distinct from the Pfizer people listed below.
What is interesting is there was A.I. input into the cardiac assessments from US2.AI – a company that specialises in this to which one of the authors Dr Solomon has links.
Author Affiliations:
Department of Cardiology, Copenhagen University Hospital–Herlev andGentofte, Copenhagen, Denmark (Lassen, Johansen, Christensen, Skaarup, Modin, A. M. R. Jensen, Dons, Bernholm, Davidovski, Duus, Ottosen, Nielsen, Borchsenius, Espersen, Köse,Fussing, Pareek, Biering-Sørensen);
Center for Translational Cardiology and Pragmatic Randomized Trials, Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (Lassen, Johansen, Christensen, Skaarup, Modin, A. M. R. Jensen, Dons, Bernholm, Davidovski, Duus,Ottosen, Nielsen, Borchsenius, Espersen, Köse, Fussing, Pareek, Biering-Sørensen);
Pfizer Inc, New York, New York (Aliabadi, Gessner, Schwarz, Gonzalez, Skovdal, Zhang, Begier);
Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts (Claggett, Solomon);
Department of Clinical Medicine–Department of Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark (C. S. Larsen);
European LifeCare Group, Søborg, Denmark (C. S. Larsen);
Research Unit for Infectious Diseases, Odense University Hospital, Odense, Denmark (L. Larsen);
Department of Infectious Diseases, Zealand University Hospital, Roskilde, Denmark (Wiese);
Department of Infectious Diseases, Aalborg University Hospital, Aalborg, Denmark (Dalager-Pedersen);
Department of Clinical Medicine, Aalborg University Hospital, Aalborg, Denmark (Dalager-Pedersen);
Department of Cardiology, Zealand University Hospital Roskilde, Roskilde, Denmark (Lindholm);
Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (Køber, J. U. S. Jensen);
Department of Cardiology, Copenhagen University Hospital–Rigshospitalet, Copenhagen, Denmark (Køber, Biering-Sørensen);
Respiratory Medicine Section, Department of Medicine, Copenhagen University
Hospital–Herlev and Gentofte, Copenhagen, Denmark (J. U. S. Jensen);
Statens Serum Institut, Copenhagen, Denmark (Martel);
Pfizer Canada, Kirkland, Quebec, Canada (Moulton);
Steno Diabetes Center Copenhagen, Copenhagen, Denmark (Biering-Sørensen).
Author Contributions: Drs Lassen and Biering-Sørensen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Lassen, Johansen, Christensen, Aliabadi, Skaarup, Davidovski, Köse, Solomon, Martel, Gessner, Schwarz, Gonzalez, Skovdal, Zhang, Begier, Biering-Sørensen.
Acquisition, analysis, or interpretation of data: Lassen, Johansen, Aliabadi, Skaarup, Modin, Claggett, C. Larsen, L. Larsen, Wiese, Dalager-Pedersen, Lindholm, A. Jensen, Dons, Bernholm, Davidovski, Duus, Ottosen, Nielsen, Borchsenius, Espersen, Köse, Fussing, Pareek, Køber, J. Jensen, Gessner, Moulton, Begier, Biering-Sørensen.
Drafting of the manuscript: Lassen, Moulton, Biering-Sørensen.
Critical review of the manuscript for important intellectual content: Johansen, Christensen, Aliabadi, Skaarup, Modin, Claggett, C. Larsen, L. Larsen, Wiese, Dalager-Pedersen, Lindholm, A. Jensen, Dons, Bernholm, Davidovski, Duus, Ottosen, Nielsen, Borchsenius, Espersen, Köse, Fussing, Pareek, Køber, Solomon, J. Jensen, Martel, Gessner, Schwarz, Gonzalez, Skovdal, Moulton, Zhang, Begier, Biering-Sørensen.
Statistical analysis: Lassen, Modin, Moulton, Zhang, Biering-Sørensen.
Obtained funding: Christensen, Gessner, Begier, Biering-Sørensen.
Administrative, technical, or material support: Christensen, L. Larsen, Dalager-Pedersen, A. Jensen, Dons, Bernholm, Davidovski, Duus, Nielsen, Borchsenius, Fussing, Martel, Schwarz, Begier, Biering-Sørensen.
Supervision: Johansen, Aliabadi, Claggett, C. Larsen, Dalager-Pedersen, Lindholm, Pareek, Køber, Solomon, J. Jensen, Gessner, Schwarz, Gonzalez, Skovdal, Biering-Sørensen.
Conflict of Interest Disclosures: Dr Aliabadi reported being an employee of Pfizer Inc and owning Pfizer stock.
Dr Skaarup reported receipt of personal fees for advisory board membership from Sanofi and travel support for congress participation from AstraZeneca.
Dr Claggett reported receipt of personal fees for consulting from Alnylam, Cardior, Cardurion, Cytokinetics, CVRx, Intellia, Rocket, Lilly, and Alexion.
Dr Pareek reported receipt of personal fees for advisory board membership and/or speakerfees from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen-Cilag, and Novo Nordisk and grants from the Danish Cardiovascular Academy and the Danish Heart Foundation.
Dr Køber reported receipt of speaker honoraria from Novo Nordisk, Novartis, AstraZeneca, and Boehringer Ingelheim.
Dr Solomon reported receipt of grants to his institution from Alexion, Alnylam, Applied Therapeutics, AstraZeneca, Bellerophon, Bayer, BMS, Boston Scientific, Cytokinetics, Edgewise, Eidos/BridgeBio, Gossamer, GSK, Ionis, Lilly, the National Institutes of Health/National Heart, Lung, and Blood Institute, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, Tenaya, Theracos, and US2.AI and personal fees from Abbott, Action, Akros, Alexion, Alnylam, Amgen, Arena, AskBio, AstraZeneca, Bayer, BMS, BridgeBio, Cardior, Cardurion, Corvia, Cytokinetics, GSK, Intellia, Lilly, Novartis, Roche, Theracos, Quantum Genomics, Tenaya, Sanofi Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, Valo, Synhale, and Recordati.
Dr Gessner, Dr Schwarz, Ms Gonzalez, Ms Skovdal, Dr Moulton, Ms Zhang, and Dr Begier reported being employees of Pfizer Inc and owning Pfizer stock.
Dr Biering-Sørensen reported receipt of personal fees for consulting and/or lectures from Sanofi Pasteur, GSK, Novo Nordisk, IQVIA, Parexel, Amgen, CSL Seqirus, Bayer, Novartis, and GE Healthcare and grants from Sanofi Pasteur, Pfizer, AstraZeneca, Boston Scientific, GE Healthcare, Novartis, Novo Nordisk, Bayer, and AstraZeneca.
No other disclosures were reported.
Funding/Support: DAN-RSV was funded by Pfizer Inc.
Role of the Funder/Sponsor: In this collaboration between the Center for Translational Cardiology and Pragmatic Randomized Trials (CTCPR) at the Department of Cardiology, Copenhagen University Hospital–Herlev and Gentofte, University of Copenhagen, Denmark, and the private vaccine provider Danske Lægers Vaccinations Service/ European LifeCare Group, the CTCPR acted as trial sponsor and the central trial coordination site and was responsible for the overall study design, conduct, registry-based recruitment and data collection, and safety monitoring.
Pfizer Inc participated in the design of the study and in the development of the protocol and statistical analysis plan. Pfizer Inc had no role in the conduct of the study; collection, management, analysis, or interpretation of the data; preparation of the manuscript; or decision to submit the manuscript for publication. Pfizer Inc was involved in reviewing and approving the final version of the manuscript.
Meeting Presentation: Presented at ESC Congress 2025; August 30, 2025; Madrid, Spain.
Data Sharing Statement: See Supplement 4.
Additional Contributions: We thank all personnel at European LifeCare Group for collaborating and assisting with study procedures.
I now realise that articles using data from this paper-mill were simultaneously published in two other journals, also on 30 August.
Commencing countdown:
We’re now up to four.
Is this a record? (Geddit?)
https://www.jacc.org/doi/10.1016/j.jacc.2025.08.023
https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehaf679/8244098?searchresult=1
Engines on
(Four, three, two) Check ignition
(One) And may God’s love
(Lift-off) Be with you
For some strange reason the name Abrysvo® is nowhere to be seen in any of these papers, which instead refer to RSVpreF vaccine.
Why would Pfizer not want the publicity?
Could it be anything to do with the higher all-cause death rate in the over 60’s?
It is not strange that Abrysvo is not mentioned. As a video in tomorrow’s post will show, the big deal for Pfizer is to occupy the only RSV vaccine ground. You want a vaccine for RSV – you almost have no option now that even Astra-Zeneca are calling Beyfortus a drug. This war is only starting.
D
Single Dose of RSV Vaccine Demonstrates Long-Term Effectiveness in Older Adults
https://themunicheye.com/single-dose-rsv-vaccine-effectiveness-older-adults-26014
‘While the study affirms the effectiveness of the vaccine, it also notes a gradual decline in its protective benefits over time. Self remarked that the diminishing efficacy suggests the possibility of needing booster doses to sustain protection against RSV in the long term. Monitoring the durability of vaccine effectiveness will be crucial for informing future vaccination strategies.’
Here we go, ‘the ‘Booster’ strategy..
You mean the make money approach?
D
Clesrovimab is made by Merck and looks very unlikely to make inroads into the RSV market. Are Merck trying to strong-arm the UK Government to including it as a mandated vaccine?
Blow for UK drugs sector as Merck scraps £1bn expansion https://www.bbc.com/news/articles/ckgzyxjr0lzo
Faarea Masud and Rachel Clun
US pharmaceutical giant Merck is scrapping a planned £1bn expansion of its UK operations, saying the government is not investing enough in the sector.
The multi-national business, known as MSD in Europe, said it would move its life sciences research to the US and cut UK jobs, blaming successive governments for undervaluing innovative medicines. One science industry expert told the BBC that, following Merck’s decision, many major pharmaceutical companies could stop investing in the UK.
A spokesperson for the government defended its investments in science and research, but acknowledged there was “more work to do”.
Pharmaceutical companies have been refocusing on investing in the US following pressure from President Donald Trump, including threats of sky-high tariffs on drug imports.
Merck had already begun construction on a site in London’s King’s Cross which was due to be completed by 2027, but said it no longer planned to occupy it. The company will also vacate its laboratories in the London Bioscience Innovation Centre and the Francis Crick Institute by the end of the year, which will lead to 125 job losses.
A spokesperson for the drug company said the decision “reflects the challenges of the UK not making meaningful progress towards addressing the lack of investment in the life science industry and the overall undervaluation of innovative medicines and vaccines by successive UK governments”.
‘Global uncertainty’
Speaking in the House of Commons, science minister Ian Murray said Merck’s decision was “deeply disappointing” but that it was “a commercial decision for them”.
The company recently announced 125 job losses and $3bn (£2.2bn) per year cost cuts.
Mr Murray said global economic pressures and the US trade policy had compounded its problems.
He added that pharmaceutical companies were getting a lower proportion of NHS drugs spending due to the actions of previous Conservative and coalition governments.
However, Conservative shadow science secretary Julia Lopez said the message from Merck bosses was “unsparing”. “Simply put, the UK is not internationally competitive”, she said. “The government must wake up, and do so now.”
Drugs spending
Sir John Bell, emeritus regius professor of medicine at Oxford University, told the BBC’s Today programme that he’d spoken to several bosses of major companies in the past six months, “and they’re all in the same space, and that is, they’re not going to do any more investing in the UK”.
One of the problems, he said, was the amount of money the NHS spends on medicines. “Ten years ago, we used to spend 15% of our healthcare spend on pharmaceuticals. Now it’s 9%. The rest of the world, the OECD, are sitting between 14 and 20%,” Sir John said. “The large companies do have to work in a system where they can sell their products, and if they can’t sell their products here, they’ll go and do their business somewhere else.”
Richard Torbett, head of the Association of the British Pharmaceutical Industry, said the decision was “an incredible blow”. “We’ve really got to see it as a wake up call to try and understand what is driving companies to make these difficult decisions and what can we do to turn that round,” he told the BBC’s Wake Up To Money programme. “The lack of competitiveness of the UK is the big thing that’s driven the decision,” he added. “We’ve got systematic under-investment in the products that come out of the end of innovation.”
MSD is the latest pharmaceutical company to abandon or reduce investment plans in the UK.
In January, AstraZeneca walked away from plans to invest £450m in expanding a vaccine manufacturing plant in Merseyside earlier this year, blaming reduced government support.
The UK boss of another pharmaceutical giant warned last month that NHS patients would lose access to cutting-edged treatments because Britain was “largely uninvestable”. Norvartis’s Johan Kahlstrom said the company had “already been unable to launch several medicines” in the country due to the “declining competitiveness” of the UK market.
In 2023, AstraZeneca chose to build a new factory in the Republic of Ireland instead of the UK. The firm’s boss said at the time it wanted to build the site in north-west England, but a “discouraging” tax rate meant it chose Dublin instead.
Industry sources told the BBC the sector had been attracting major funding in the hub around Kings Cross focused on the intersection between life sciences and AI.
They pushed back on claims that the decision was linked to ongoing negotiations over drug prices, in which industry has been lobbying hard for the NHS to approve more and pay more for medicines. The current pricing regime was set and agreed to by drug companies in 2023 – less than 18 months ago.
Since then, pharmaceutical firms have come under pressure from the Trump administration to lower drug prices for American customers and to invest more in the US – affecting their ability to invest elsewhere.
In an August interview with CNBC, Trump suggested that tariffs on pharmaceuticals imported to the US could reach up to 250%. The threat followed an executive order signed by the president in May aimed at reducing drug prices for American consumers.
Dr David Roblin, chief executive of London-based biotechnology company Relation Therapeutics, told the BBC the fundamentals that drove MSD to invest in the UK in the first place had not changed.
“The academic environment in the UK continues to produce innovative ideas and people to run with those ideas, which attracts foreign investment,” he said.
“The environment to do research is still outstanding: we’ve got great academics, the NHS does provide a research platform, for example the UK Biobank is proving to be a real attractor for companies like mine,” he said.
What has changed, Dr Roblin said, was the political landscape in the US which big pharma has to respond to, “because the US remains the largest market for pharmaceuticals on earth,” he added.
A spokesperson for the Department of Industry, Science and Technology said: “The UK has become the most attractive place to invest in the world, but we know there is more work to do. “We recognise that this will be concerning news for MSD employees and the government stands ready to support those affected.”
In Labour’s election manifesto, the party said that as part of its life sciences plan it would develop “an NHS innovation and adoption strategy in England”. “This will include a plan for procurement, giving a clearer route to get products into the NHS coupled with reformed incentive structures to drive innovation and faster regulatory approval for new technology and medicines,” it said.
This is an unsurprising situation. There are very few innovative products being produced that save lives or enhance function. There are knock-off copies of older drugs. There are ‘vaccines’ which companies hope governments with force entire populations to take. There is no surprise that this scenario leads to threats. If only opportunistic threats – taking advantage of a bad situation in terms of products anyone really wants to put pressure on politicians and others.
D