One of the hopes of this blog is to create a repository of maneuvers through which clinical trials can be gamed to get results. The series of posts laying out some of the less well known tricks are filed under the Hiding the Bodies blog category. To be more generally useful, this repository needs others to contribute further maneuvers to make it comprehensive and to contribute examples from areas of medicine other than mental health to help people more generally to spot what is going on. I will add to the lists below as examples come in.
After this post, (and after a pharma-myth and personal account), the next series of posts will tackle the notion that randomized controlled trials (RCTs) offer a Gold Standard when it comes to determining what drugs cause. The argument in brief will be that RCTs provide something close to an ultimate, and at present unrecognized, bias behind which companies can hide bodies. These posts will be filed under the Spin & Data blog category.
Clinical trial maneuvers typically listed in the literature include:
- Switching endpoints after the results come in
- Surrogate rather than substantive outcome measures
- Inappropriate use of Number Needed to Treat (NNT)
- Last Observations Carried Forward (LOCF)
- Relative Risks: results expressed in relative rather than absolute risks
- Short term trials, when long term administration is contemplated
- Co-administration of agents that will minimize side effects
- Publication management: non-publication, ghostwriting of publications, etc.
Recent posts have demonstrated a series of less commonly listed strategies:
- Moving bodies to boost apparent risks in the placebo group
- Creating doubt – using statistical significance to hide risks
- Splitting hazards so they appear under different coding groups
- Inappropriate use of duration of treatment to hide risks
- Running trials with high baselines of adverse events to drown out hazards
- Actively withholding trials from regulators (this can be done quite legally – the regulator has to ask for exactly the right thing, and regulators encouraged to partner industry are perhaps less likely to get the questions right)
- Companies commonly use placebo washout periods to get rid of placebo responders.
- Companies also use washout weeks to eliminate anyone having an adverse response to the active treatment.
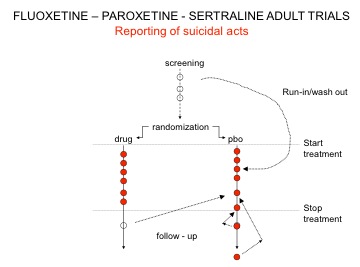
Here is a variant on maneuver 1 outlined in Drug companies use studies the way a drunk uses a lamppost where the placebo washout was used by Lilly, GSK, and Pfizer to hide suicidal acts. When reviewing the issue of antidepressants and suicide in 2003, the MHRA asked for, but didn’t get, all trials and asked companies not to repeat this placebo washout maneuver.
Unable to move bodies from the washout phase, GSK instead took three suicides from the post-trial period and filed them under placebo – see Figure 1.
- After his trial was over one of the patients had been put on clotiapine, oxazepam, and fluoxetine. He went on to commit suicide, and this Prozac-treated patient was coded as a placebo suicide.
- A second killed himself on day 33 after the trial was over. The post-trial period at the end of these GSK trials was a 30-day period. This patient shouldn’t have been included.
- The third patient might have committed suicide 19 days after completing the study, but all that is filed is a brief mention that the patient’s brother called to report the death with no confirmation of cause of death. We don’t know if this patient was also put on an SSRI and then committed suicide.
A good case can be made for excluding one, or two, or all three of these suicides. But part of the mystery is this: when there was only one suicide on Paxil and none on placebo in these trials, one suicide in about 34,000 months of study, why should three turn up in this withdrawal month, all from the placebo group? It is a statistical freak.
In the face of this statistical freak why…
In the face of this freak why did the regulator (MHRA) include these suicides in the mix, helping in the process to hide the risks not just on Paxil but on all SSRIs?
Did the MHRA think that just because they had told companies not to import suicides from the washout period that this would bring an end to all efforts to find bodies elsewhere?
Why did David Gunnell, Julia Saperia, and Deborah Ashby, who produced an article for BMJ (19 February 2005) on the data from antidepressant trials and suicide, include these three placebo suicides?
When details of the suicides were drawn to their attention, why did DG/JS/DA do nothing about it?
Why did no one at BMJ spot the issue?
Figure 1
CITATION DISTORTION IS ANOTHER TRICK.
Most people are now aware how the academic literature on medications is undermined, (perhaps fatally for some drug classes such as antidepressants [1]), by publication bias and selective reporting. To these, we must now add citation distortion or “unfounded authority”. Unfortunately, much consensus in psychopharmacology is based on unfounded authority.
Citation distortion [2,3] is the process whereby, in a field with ambiguous primary data, studies showing the desired outcome are preferentially cited. Over years and generations of reviews, this distortion is amplified with exponential increases in supportive citation, facilitated by increased citation of papers that do not contain primary data (ie, one review cites another). At the same time, there is no parallel increase in citation of critical reviews.
Errors accumulate in this process of reiterative citation; for example what is acknowledged as hypothesis in cited articles is misrepresented as fact in reviews.
An example from this week’s academic literature is an article from Australia’s MJA [4]. Amongst the numerous self-citations (count them), note the disingenuous citation of the discredited Gibbons et al paper (see David’s blog at https://davidhealy.org/coincidence-a-fine-thing) as though it supported ‘The importance of managing anxiety and depression in young people to minimise functional, medical and psychological complications’.
[1] Ioannidis JP. Effectiveness of antidepressants: an evidence myth constructed
from a thousand randomized trials? Philos Ethics Humanit Med. 2008 May
27;3:14. http://peh-med.com/content/pdf/1747-5341-3-14.pdf.
[2] Greenberg S. How citation distortions create unfounded authority: analysis of a citation network. BMJ2009;339doi: 10.1136/bmj.b2680 (Published 21 July 2009) http://www.bmj.com/content/339/bmj.b2680.full
[3] Slawson DC, Shaughnessy AF. Obtaining useful information from expert based sources. BMJ. 1997;314(7085):947-9. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2126390/pdf/9099121.pdf
[4] Scott EM et al. Targeted primary care-based mental health services for young Australians. MJA 2012; 196: 136-140 http://www.mja.com.au/public/issues/196_02_060212/sco10481_fm.html
I’m not sure where this post might rightly belong but it’s something I believe should be seen by everyone interested in the actions of Big Pharma – especially women. http://www.youtube.com/watch?v=TUY-iTf2T1A is the URL for the trailer for a hilarious but terrifying documentary about the development of Cialis and the creation of yet another disease – female sexual dysfunction. The film, called “Orgasm.inc” is available on Netflix in the US. The DVD is on Amazon. If you want to laugh while crying and cringing, please watch it. The trailer alone is worth a viewing.