
The Letter below from Marty Keller and colleagues was sent to many media outlets, to retraction watch, and to professional organizations on Wednesday. Paul Basken from the Chronicle for Higher Education asked me for a response which I sent about an hour after receiving the letter. This response is from me rather than the 329 group. This and other correspondence features and will feature on Study329.org.
One quick piece of housekeeping. Restoring Study329 is not about giving Paroxetine to Adolescents – it’s about all drugs for all indications across medicine and for all ages. It deals with standard Industry MO to hype benefits and hide harms. One of the best bits of coverage of this aspect of the story yesterday was in Cosmopolitan.
Letter from Keller et al
Dear
Nine of us whose names are attached to this email (we did not have time to create electronic signatures) were authors on the study originally published in 2001 in the Journal of the American Academy of Child and Adolescent Psychiatry entitled, “Efficacy of paroxetine in the treatment of adolescent major depression: a randomized controlled trial,” and have read the reanalysis of our article, which is entitled, “Restoring Study 329: efficacy and harms of paroxetine and imipramine in treatment of major depression in adolescence”, currently embargoed for publication in the British Medical Journal (BMJ) early this week. We are providing you with a brief summary response to several of the points in that article that which with we have strong disagreement. Given the length and detail of the BMJ publication and the multitude of specific concerns we have with its approach and conclusions, we will be writing and submitting to the BMJ’s editor an in-depth letter rebutting the claims and accusations made in the article. It will take a significant amount of work to make this scholarly and thorough and do not have a time table; but that level of analysis by us far exceeds the time frame needed to give you that more comprehensive response by today.
The study was planned and designed between 1991-1992. Subject enrollment began in 1994, and was completed in 1997, at which time analysis of the data commenced. The study authors comprised virtually all of the academic researchers studying the treatment of child depression in North America at the time. The study was designed by academic psychiatrists and adopted with very little change by GSK, who funded the study in an academic / industry partnership. The two statisticians who helped design the study are among the most esteemed in psychiatry. The goal of the study designers was to do the best study possible to advance the treatment of depression in youth, not primarily as a drug registration trial. Some design issues would be made differently today — best practices methodology have changed over the ensuing 24-year interval since inception of our study.
In the interval from when we sat down to plan the study to when we approached the data analysis phase, but prior to the blind being broken, the academic authors, not the sponsor, added several additional measures of depression as secondary outcomes. We did so because the field of pediatric-age depression had reached a consensus that the Hamilton Depression Rating Scale (our primary outcome measure) had significant limitations in assessing mood disturbance in younger patients. Accordingly, taking this into consideration, and in advance of breaking the blind, we added secondary outcome measures agreed upon by all authors of the paper. We found statistically significant indications of efficacy in these measures. This was clearly reported in our article, as were the negative findings.
In the “BMJ-Restoring Study 329 …” reanalysis, the following statement is used to justify non-examination of a range of secondary outcome measures:
Both before and after breaking the blind, however, the sponsors made changes to the secondary outcomes as previously detailed. We could not find any document that provided any scientific rationale for these post hoc changes and the outcomes are therefore not reported in this paper.
This is not correct. The secondary outcomes were decided by the authors prior to the blind being broken. We believe now, as we did then, that the inclusion of these measures in the study and in our analysis was entirely appropriate and was clearly and fully reported in our paper. While secondary outcome measures may be irrelevant for purposes of governmental approval of a pharmaceutical indication, they were and to this day are frequently and appropriately included in study reports even in those cases when the primary measures do not reach statistical significance. The authors of “Restoring Study 329” state “there were no discrepancies between any of our analyses and those contained in the CSR [clinical study report]”. In other words, the disagreement on treatment outcomes rests entirely on the arbitrary dismissal of our secondary outcome measures.
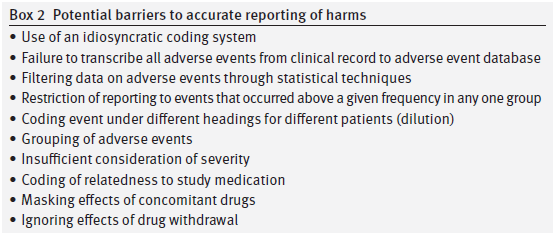
We also have areas of significant disagreement on the “Restoring Study 329” analysis of side effects (which the author’s label “harms”). Their reanalysis uses the FDA MedDRA approach to side effect data, which was not available when our study was done. We agree that this instrument is a meaningful advance over the approach we used at the time, which was based on the FDA’s then current COSTART approach. That one can do better reanalyzing adverse event data using refinements in approach that have accrued in the 15 years since a study’s publication is unsurprising and not a valid critique of our study as performed and presented.
A second area of disagreement (concerning the side effect data) is with their statement, “We have not undertaken statistical tests for harms.” The authors of “Restoring Study 329” with this decision are saying that we need very high and rigorous statistical standards for declaring a treatment to be beneficial but for declaring a treatment to be harmful then statistics can’t help us and whatever an individual reader thinks based on raw tabulation that looks like a harm is a harm. Statistics of course does offer several approaches to the question of when is there a meaningful difference in the side effect rates between different groups. There are pros and cons to the use of P values, but alternatives like confidence intervals are available.
“Restoring Study 329” asserts that this paper was ghostwritten, citing an early publication by one of the coauthors of that article. There was absolutely nothing about the process involved in the drafting, revision, or completion of our paper that constitutes “ghostwriting”. This study was initiated by academic investigators, undertaken as an academic / industry partnership, and the resulting report was authored mainly by the academic investigators with industry collaboration.
Finally the “Restoring Study 329” authors discuss an initiative to correct publications called “restoring invisible and abandoned trials (RIAT)” (BMJ, 2013; 346-f4223). “Restoring Study 329” states “We reanalyzed the data from Study 329 according to the RIAT recommendations” but gives no reference for a specific methodology for RIAT reanalysis. The RIAT approach may have general “recommendations” but we find no evidence that there is a consensus on precisely how such a RIAT analysis makes the myriad decisions inherent in any reanalysis nor do we think there is any consensus in the field that would allow the authors of this reanalysis or any other potential reanalysis to definitively say they got it right.
In summary, to describe our trial as “misreported” is pejorative and wrong, both from consideration of best research practices at the time, and in terms of a retrospective from the standpoint of current best practices.
Martin B. Keller, M.D.
Boris Birmacher, M.D.
Gregory N. Clarke, Ph.D.
Graham J. Emslie, M.D.
Harold Koplewicz, M.D.
Stan Kutcher, M.D.
Neal Ryan, M.D.
William H. Sack, M.D.
Michael Strober, Ph.D.
Response
In the case of a study designed to advance the treatment of depression in adolescents, it seems strange to have picked imipramine 200-300mg per day as a comparator, unusual to have left the continuation phase unpublished, odd to have neglected to analyse the taper phase, dangerous to have downplayed the data on suicide risks and the profile of psychiatric adverse events more generally and unfortunate to have failed to update the record in response to attempts to offer a more representative version of the study to those who write guidelines or otherwise shape treatment.
As regards the efficacy elements, the correspondence we had with GSK, which will be available on Study329.org as of Sept 16 and on the BMJ website, indicates clearly that we made many efforts to establish the basis for introducing secondary endpoints not present in the protocol. GSK have been unwilling or unable to provide evidence on this issue, even though the protocol states that no changes will be permitted that are not discussed with SmithKline. We would be more than willing to post any material that Dr Keller and colleagues can provide.
Whatever about such material, it is of note that when submitting Study 329 to FDA in 2002, GSK described the study as a negative Study and FDA concurred that it was negative. This is of interest in the light of Dr Keller’s hint that it was GSK’s interests to submit this study to regulators that led to a corruption of the process.
Several issues arise as regards harms. First, we would love to see the ADECs coding dictionary if any of the original investigators have one. Does anyone know whether ADECs requires suicidal events to be coded as emotional lability or was there another option?
Second, can the investigators explain why headaches were moved from classification under Body as a Whole in the Clinical Study Report to sit alongside emotional lability under a Nervous System heading in the 2001 paper?
It may be something of purist view but significance testing was originally linked to primary endpoints. Harms are never the primary endpoint of a trial and no RCT is designed to detect harms adequately. It is appropriate to hold a company or doctors who may be aiming to make money out of vulnerable people to a high standard when it comes to efficacy but for those interested to advance the treatment of patients with any medical condition it is not appropriate to deny the likely existence of harms on the basis of a failure to reach a significance threshold that the very process of conducting an RCT will mean cannot be met as investigators attention is systematically diverted elsewhere.
As regards RIAT methods, a key method is to stick to the protocol. A second safeguard is to audit every step taken and to this end we have attached a 61 page audit record (Appendix 1) to this paper. An even more important method is to make the data fully available, which it will be on Study329.org.
As regards ghostwriting, I personally am happy to stick to the designation of this study as ghostwritten. For those unversed in these issues, journal editors, medical writing companies and academic authors cling to a figleaf that if the medical writers name is mentioned somewhere, s/he is not a ghost. But for many, the presence on the authorship line of names that have never had access to the data and who cannot stand over the claims made other than by assertion is what’s ghostly.
Having made all these points, there is a point of agreement to note. Dr Keller and colleagues state that:
“nor do we think there is any consensus in the field that would allow the authors of this reanalysis or any other potential reanalysis to definitively say they got it right”.
We agree. For us, this is the main point behind the article. This is why we need access to the data. It is only with collaborative efforts based on full access to the data that we can manage to get to a best possible interpretation but even this will be provisional rather than definitive. Is there anything that would hold the authors of the second interpretation of these data (Keller and colleagues) back from joining with us the authors of the third interpretation in asking that the data of all trials for all treatments, across all indications, be made fully available? Such a call would be consistent with the empirical method that was as applicable in 1991 as it is now.
David Healy
Holding Response on Behalf of RIAT 329
From the GSK ‘Code of Conduct’ on their website:
Is this true? Re CSRs?
“For human subject research that evaluates medicines, we post protocol
and result summaries on internet registers. We also post the protocols and clinical study reports with the removal of personally identifiable information. “
Nice to see the guys in panic mode…………. Lets look forward to seeing if their comments are worth the space in British Medical Journal. From the contents of the letter, I would say probably not but I hope they write something better than this letter.
Well done, to you all………… you have obviously been prepared, very, very well…….. Yes I hope you can debunk all the other trials, there are so many, it is so sad, that they are willing to murder, for addiction and pure greed from their failed products.
And psychiatrists? They frighten people, so they have this sort of undeserved power, without a court, they can label you, they can send you mad on medication, and all the time claim how wonderful they are……….
Wait a minute. Are these guys complaining because you applied statistical tests to determine whether improvements in the teens’ moods were really on account of Paxil – but you didn’t apply statistical tests to make sure the suicide attempts were really on account of Paxil? I’m all right with that. I think it’s the right thing to do. If only 7% of patients are helped by a certain pill, that’s not interesting; don’t bother to publish it. But if “only” 7% of patients are seriously harmed by a pill, that should be VERY interesting.
As I understand it, “statistical significance” involves being 95% certain that the changes you see in the people taking pills would not have just happened naturally. Even very small improvements can be “significant” if you are very sure they’re drug-related. You might need to treat lots of people to see them, though.
These tests make some sense if the changes are not huge, and the illness is usually limited, and there are other ways to treat it already. (They might not make sense if the disease is usually fatal and there are no decent treatments yet.) You don’t want to flood the market with new chemical treatments if you’re not really sure they do any good. This probably applies to depression as experienced by the kids in the study.
They make NO sense when you’re dealing with serious side effects of treatment, for a disease that often gets better on its own. If eleven kids become “suicidal” in a serious way on the drug, and only one on placebo, you may not have 95% certainty. But in my book, even 60% certainty would be enough to say WHOA! Even 30% “certainty”, which is only an inkling, would be worth mentioning in the article. If the pill is approved, your colleagues should still keep an eye out for this.
The Hippocratic Oath, which doctors have repeated for centuries, famously says “First do no harm.” It does not say, “Go out there and mess around, and if you think you may have caused some harm, but you’re pretty sure you caused even more good, then congratulations! You’re a helluva doctor!”
Is Keller aware of that ALL doctors (99,8%) use data from RCT’s to say: “that is an anecdote because RCT’s did not show that”?
Is Keller aware of that almost no RCT’s ever produced has had emphasis on finding possible adverse events, but rather to find enough evidence to ensure economic success for the manufacturer?
Is Keller aware of that I saw him trembling infront of the FBI investigators, not remembering to have ever seen any data they were asking for?
(But now, all of a sudden, he has the “cojones” to write a reply to DH with such confidence?)
“If you can’t speak with confidence in Court, don’t bring your lack of rethoric to the schoolyard to bully others” -Ove 2015.
Reply to Keller et al.
If Dr. Keller was principal investigator in study 329, wasn’t he responsible for verifying the data? Dr. Keller stated in his sworn deposition that he did not do so because he doesn’t like to review pages and pages of numbers. This, of course, distinguishes the authors of the 2001 JAACAP paper from those who devoted themselves to the reanalysis of study 329. At least, Le Noury et al. studied the patient-level data rather than trusting GSK’s statisticians. The RIAT reanalysis is a pioneering work that paves the way for similar treatment of all industry-sponsored clinical trials published in the medical journals.
In Paul Basken’s article “Landmark Analysis of an Infamous Medical Study Points Out the Challenges of Research Oversight,” in The Chronicle of Higher Education on September 17, 2015, Dr. Keller responds to criticism of the reporting of study 329, but instead of addressing legitimate points of criticism, he attacks alleged motives of critics in an ad hominem circumstantial fallacy.
In the letter posted above, Dr. Keller and eight of his colleagues named as ‘authors’ of Keller et al., defend the design, conduct and reporting of their report, but in the Chronicle article Dr. Keller suggests “any misrepresentations of his article to help sell Paxil was the responsibility of Glaxo.” The problem, however, with academic-industry partnerships, such as the one that produced study 329, is that marketing and science are conflated and the academics become little more than expensive drug reps. Drs. Keller, Ryan and Wagner gave industry-prepared talks as continuing medical education and conference presentations on the misrepresented results of study 329.
Regarding his denial of ghostwriting, Keller et al is not only ghostwritten, but it is now the standard by which all other cases of ghostwriting are judged. Has Dr. Keller inspected the documents that are posted on Healthy Skepticism and Drug Industry Document Archive (DIDA)? If not, I would urge him to become reacquainted with these, and remind him that these are only a fraction of the declassified documents from litigation. Would Dr. Keller care to request from GSK that the documents designated as confidential be made public as well?
In the interest of open, academic debate on scientific results, I look forward to Dr. Keller’s promise of a “scholarly and thorough” analysis of the BMJ article.
Leemon McHenry
Amongst all these brief statements is one in which ‘Keller is a real piece of work’ and a few other things…about his tenure at Brown University and subsequent income from pharmaceutical companies.
When he actually touched Shelley Jofre, and, put his finger under her chin, it was repeated as Shelley was so shocked, I think at that precise moment we might have got the measure of this man.
David has been diplomatic, he mostly is, but, munchkins, do we need this nine person letter, which, as, far, as, I, can, read, is, a very poor nine-centred approach to denial that I have ever seen….it reminds me of the letters I received from my hospital and surgery…tickly under there…definitely…
https://truthman30.wordpress.com/2015/09/18/the-real-world-damage-of-gsks-notorious-seroxat-paxilaropax-drug/
If Shelley had been on Seroxat, at the time, her knee might have risen and caused, maybe, just a little painful expression…..:)
Dear 9 men of straw
What happened to all the female authors on the study 329 authorship….are they too ashamed to now be involved in something that has resulted in the death of many children and ruination of many more.
Are they too embarassed to be associated with this strange unusual odd and dangerous ….lets face it fraudulent criminal disgraceful morally repugnant murderous piece of greed.
I would like to hear from:
RACHEL G. KLEIN, PH.D.
GABRIELLE A. CARLSON, M.D.
BARBARA GELLER, MD
KAREN DINEEN WAGNER, M.D.
ELIZABETH B. WELLER, M.D
NANCY C. WINTERS, M.D.
ROSEMARY OAKES, M.S.
and then there is the spooky
SILLY LADEN
oh and lets not forget the gatekeeper:
MINA DULCAN
Your silence is deafening!
paroxetine victim.
One of the women has died – Elizabeth Weller, from cancer a few years ago. She was a no-nonsense clinical investigator who contributed patients to the study but who was probably dialed out of access to the data. I suspect that was true for a number of the listed academic authors. Back in those days the practice was for the corporation to declare ownership of the data. At best, individual investigators might get access to data just from their own site. I was always uncomfortable with the notion that site investigators were granted scientific authorship. It may have seemed like a win-win arrangement but some of these listed authors I am sure wish they had never consented to be so trusting.
http://www.pulsetoday.co.uk/clinical/mental-health/paroxetine-causes-harm-in-adolescents-researchers-claim/20020122.article#.VfxjJrS4nVo
Sure you’ve spotted this – just in case not.
I looked at the altometrics (?) data on the BMJ for the article – and it’s on there (plus a couple of others).
Apologies if you know about it already.
I must say the Alltrials response takes some beating
And thanks for the reminder that this matters for all drugs, for all people, for all ages
Thanks, again, for all you and the team have done, and are continuing to do
It makes no difference whether the failures of reporting in the 2001 GSK 329 paper by Keller et al resulted from deliberate dissembling and spin or from incompetent tradecraft. Either way, why should we now trust their scientific credentials? And why should we place trust in the beneficence of GlaxoSmithKline? That is a clear lesson of this RIAT re-analysis.
I do think it matters whether this was due to ignorance (incompetent tradecraft) or malicious intent (deliberate dissembling and spin)– just as motive, intent are always sought when prosecuting a crime of this magnitude.
Unless those who should care, do care and proceed to the next level of exposure and prosecution, only the *we* to which you refer are on safer ground.
What is the estimated head count in this *we* category? What percent of the population can draw relevant to their personal lives, conclusions based on some MSM discussion of those *contentious, prominent academics* who are always at each other’s ego driven throats. How many people routinely read, and research the information on this and the few other credible sites reporting the reasons we should rethink our trust in the scientific credentials of those who have the power and authority to kill us?
More to the point: What makes you think that Keller et al and/or GlaxoSmithKline give a rat’s a** , that *we* no longer trust their scientific credentials?
So far, I’d say the clear lesson of this RIAT analysis is that corrupt, profit driven, Market Based Medicine, kills– is life threatening at the very least.
Film at eleven? Not even…
In regards to the “9 men of straw’s” response…
These folks were used by GSK. It’s classic GSK behavior. They use these academics the same way the Columbian cartels use the middle men drug pushers. They’re the ones who push the drugs to the ones lower on the chain. It’s a hierarchy. GSK are the cartel, untouchable, in their ivory towers, the academic psychiatrists are the middle men who push the drugs on the small time street corner dealers (the GP’s and doctors). The doctors dispense the drugs to the addicts (the patients) and GSK never ever get touched. They operate above the law. These academics got caught, they got burned, they should be careful of the devil that they bargain with. GSK are ruthless, callous and utterly sociopathic.
Around the time that Paroxetine was being pushed off label on kids and adults for all sorts of things, and around the time that this bogus 329 study was doing the rounds, GSK were also paying other doctors, such as Dr Drew Pinksy, they were also pushing many of their other drugs to doctors, encouraging them to prescribe those drugs off label. They had thousands of docs on the payroll pushing various drugs, suppressing all sorts of problems- they still do this.
Study 329 was just the incident that caused some of the most scandalous controversy for GSK ( dead kids tend to raise eyebrows) but if you read through Greg Thorpe’s Whistleblower Complaint, you will see that Study 329 was but a small part of GSK’s unethical shenanigans. When all of these study 329 authors tell us how much money that have personally gained from their services to drug companies over the years, then perhaps maybe then we can weight up their rebuttals, until then I say…. hogwash… B.S.
Hopefully RIAT will be cited in a hundred years. Greg Thorpe is another hero. They’re thin on the ground…..
https://truthman30.wordpress.com/2015/08/28/whistleblower-greg-thorpes-7th-ammended-complaint/
@truthman,
I think you are wrong assuming that :
” It’s classic GSK behavior. They use these academics the same way the Columbian cartels use the middle men drug pushers.”
These prominent academics (mostly psychiatrists) are not patsies– they have *used GSK, J&J, et al..* to further their own narcissistic career goals–
Name one of these academics who is claiming he/she was *used*?
and below to: Bob Fiddaman,
Are you blocking out the significance of a prominent academic psychiatrist (Keller)employing Goebbels’s mantra — “If you tell a lie big enough and keep repeating it, people will eventually believe it.”–in concert with *most* pharmaceutical companies?
Is there NOT a difference between a corporate business shark and a physician? In terms of standards of conduct?
And how freaking difficult is it to establish that the *lies* are spun from their warped minds and not remotely based on science, much less rational human thought? Science and rational human behavior are the guidelines, the standards of their professions. They can and should be held accountable to established guidelines.
Keller isn’t lying about his age, and GSK is not lying about their Swiss bank accounts. Enough with the Monday morning quarterbacking , the psychobabble and the cheap excuses for *exploiting vulnerable kids for profit*
How important is it to diagnose the psychopathology of *human traffickers* at the height of their success ?
Willingly ‘used’ I should have said..
Sold their souls, reputations and patients’ lives and health for 30 pieces of silver…
First rule of corporate corruption, say nothing and let the dust settle.
Keller here is, it appears, speaking on behalf of a handful (small class size) of the original authors.
I’m surprised he’s broken his silence. His stance on the ghostwriting issue is one of complete and utter denial. As Leemon points out (comment above) the evidences of the ghostwriting are posted on Healthy Skepticism and Drug Industry Document Archive (DIDA). Maybe Keller has mentally blocked out any wrong-doing on his part or maybe he is using the Goebbels mantra on himself, “If you tell a lie big enough and keep repeating it, people will eventually come to believe it.” – A mantra that most pharmaceutical companies seem to use.
Whatever reason Keller now speaks out is a welcome one. Someone here refers to “Alltrials takes some beating” – Alltrials asked for my feedback – I gave it to them in detail about Pharma illegal trials of torture – of pharma drugs adverse reactions cover up in UK and Ireland and I know now also Europe – Alltrials responded by referring me to Agencies in Ireland who were already complicit in the cover up. The ghostwriting continues no matter what the pharma or what the drug or indeed what the medical device – Look up Morcellators and what they do despite the black box warning from the FDA.
Please wake up World – Pharma are only interested in their profit margins whilst many are in deep pain, in torture from the drugs of those Giants of Pharma who simply don’t give a damn for human life – the profit margins that is what should be written on the Tombstones of all who have been driven to death because of Corporate crime.
Teri,
It isn’t Pharma we need to open our eyes to– all global corporations run on greed and apathy the for human suffering that fuels their profit margins.
Men and women with degrees in medicine, holding prominent academic positions in America’s most prestigious universities *don’t give a damn about human life*
This is one clear example of too many to contemplate– the RIAT report on Paxil Study 329:
1) Evidence of life threatening adverse effects of Paxil were discovered in hidden data– patient clinical reports.
2)Evidence of life threatening adverse effects recoded to detract from their negative influence on the *safety* of the drug, Paxil were also discovered.
3) Evidence that the cherry picked prominent academics, all 22 of them, did not see or insist on seeing ALL the clinical data of this trial–AT any time– even when this RCT was critically challenged by other prominent psychiatrists– even though the reason for challenging the trial report was *protecting children from potentially life threatening adverse effects of the drug Paxil*.
4) The AANP committee who was assigned the task of reviewing study 329 –ALso made up of prominent academic psychiatrists, did not request ALL of the clinical data, but sought irrelevant statistics to uphold the unproven, challenged
results of a trial that increased prescriptions of Paxil to kids in the U.S. in the millions– Any concern shown here for the significance of the risks for rubber stamping this trial as *safe*– and endorsing the use of Paxil in children?
5) The COI, payoffs, profit as motive connections have been well documented in the group of academics who are *still* unconcerned with the findings of the RIAT report. And still using their weakest link, Keller, to make their cheap excuses for their piss poor performance as Doctors.
I am less inclined to invest energy toward a fools errand, like trying to get a leopard to change it’s spots. There is no threat from this crafty beast that we know pharma to be without their minions, prescription writers– pushing their drugs. Doctors propagating treatments using marketing strategies. Doctors accepting tainted, potentially dangerous literature in their professional journals. Doctors taking money for promoting this to their colleagues as *standards of care*. Doctors silently condoning– even if not actively participating in exploiting vulnerable people for profit. This is what we need to wake up and smell!!
What has to be the single most important discovery of the RIAT report on Paxil Study 329, is the pitiful BS response from Keller; that his consorts chose him as their spokesman, and it being little more than a topic for academic psychological analysis.
You would think S a S invented Clinical Trial Transparency according to their Twitter….Feeds
Recent Tweets
Sense About Science @senseaboutsci
Publication of previously hidden trial data allows for reanalysis of controversial ‘Study 329’ results http://bit.ly/1izM7X7 #AllTrials
What have they actually done apart from a Mass Propaganda Machine?
Alltrials have raised thousands and thousands of £/$s.
Are their customers getting value for money?
See
http://www.baumhedlundlaw.com/rons-rants/twenty-first-century-snake-oil-salesman/
My favourite on 1BOM:
Catalyzt
September 20, 2015 | 12:35 AM
Just as a general point of strategy, another approach would have been to open the response with a direct assault on Keller’s weakest and most astonishing statement:
“The RIAT approach may have general “recommendations” but we find no evidence that there is a consensus on precisely how such a RIAT analysis makes the myriad decisions inherent in any reanalysis nor do we think there is any consensus in the field that would allow the authors of this reanalysis or any other potential reanalysis to definitively say they got it right.”
I thought Mickey answered this best in his post on Thursday:
“In our RIAT Team’s reanalysis of Study 329, we had decided to follow the a priori protocol, which meant sticking to the protocol defined outcome variables and ignoring those later exploratory variables…”
RIAT used the scientific method, the authors of the study… well, they wound up doing something else.
Keller seems to be throwing up a barrage of different criticisms of the report, as if all of them were equally important. There’s a very natural impulse to respond in kind, to jump down that rabbit hole.
Yet another approach would be to simply not respond to Keller’s letter at all. The report is very strong, it has generated tremendous interest and publicity. Keller letter is weak; he sounds defensive and circumlocutious.
The danger is that policymakers, legislators, students, and busy clinicians might be lulled into the illusion that this is an academic debate about arcane points in methodology.
If I were doing PR for GSK right now, or if my name were on the original study? That’s exactly what I would want anyone following this debate to believe.
What is impressive in the Keller letter is how well [some] of his memory appears to have returned. If you have yet to see “Big Pharma Big Money : Documentary on the Money and Corruption of Big Pharmaceutical Companies.”, use this link to get to it: https://www.youtube.com/watch?v=iSA_itmEr5s Once there, go to the time point, 1.04.02. There you will see Keller being disposed under oath. Interesting, while this was years ago, he appeared to have no memory of anything associated with S329.
[A cautionary note: To the extent you are empathic, you will likely find this part of the film sad.]
Disposed – a cross between deposed and diagnosed? Or perhaps disposed.
I think >decomposed <
and what a mess !
Yes in that video Keller seems to have developed severe memory loss when he was questioned about study 329…
We are getting very good at analysis as trainee psychologists, watching their body language and the give away in the expression in the eyes…..classic ‘shifty’, we might surmise……………..