Won’t get fooled again outlined a stunning propaganda coup by GSK. On the back of a campaign for open access to clinical trial data that has drawn its inspiration from efforts by the Cochrane Tamiflu reviewers to get access to Roche’s clinical trial data, Andrew Witty came out and proclaimed that GSK were all in favor of access to clinical trial data. The BMJ threw its hat in … [Read more...] about Access to RxISK Data: Conflicts of Interest
Archives for November 2012
Won’t Get Fooled Again? GlaxoSmithKline and Access to Data
On November 22nd the European Medicines’ Agency (EMA) is holding a workshop on access to the data from clinical trials. While there have been many efforts by many people over the years to make the clinical trial process more transparent, the EMA workshop has come about primarily following the efforts of Peter Gøtzsche of the Danish Cochrane Group and Peter Doshi and Tom … [Read more...] about Won’t Get Fooled Again? GlaxoSmithKline and Access to Data
The St Bartholomew’s Day Massacre: Protestant Patients, Catholic Drugs
Margot's lover in La Reine Margot was one of the Huguenots who survived the massacre set in train by her brother Charles IX on St Bartholomew's Day in Paris in 1572. There are many politicians, bureaucrats, doctors and others, the Royalists, in a position to make a difference who know that psychotropic drugs can cause suicide or other serious problems but who instead attempt … [Read more...] about The St Bartholomew’s Day Massacre: Protestant Patients, Catholic Drugs
La Reine Margot: Data access, ghostwriting, suicide and mad reviewers
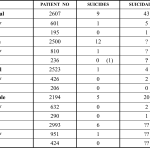
Another study giving a first hint of the findings in our 2012 Mortality in Schizophrenia paper (See The Madness of Psychiatry) was published in the British Journal of Psychiatry in 2006 - Lifetime Rates of Suicide in Schizophrenia. It took several years and some smuggling to get it into print. In the course of exploring the issues, it seemed useful to touch base with Herb … [Read more...] about La Reine Margot: Data access, ghostwriting, suicide and mad reviewers
Benefit Risk Madness: Antipsychotics and Suicide
Following the posting of The Madness of Psychiatry, there has been a flurry of activity in the twittersphere with Louis Appleby, the UK's suicide czar posting: What makes adolescents act on suicidal thoughts? New paper shows psychotic symptoms increase risk 20-fold. archpsyc.jamanetwork.com/article.aspx?a… You might get the impression from this that all patients have to do … [Read more...] about Benefit Risk Madness: Antipsychotics and Suicide